Keto-enol tautomerism
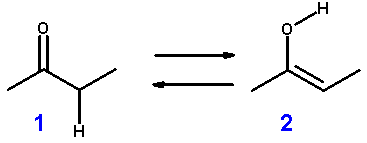
In organic chemistry, keto-enol tautomerism refers to a chemical equilibrium between a keto form (a ketone or an aldehyde) and an enol. The enol and keto forms are said to be tautomers of each other. The interconversion of the two forms involves the movement of a proton and the shifting of bonding electrons; hence, the isomerism qualifies as tautomerism.
A compound containing a carbonyl group (C=O) is normally in rapid equilibrium with an enol tautomer, which contains a pair of doubly bonded carbon atoms adjacent to a hydroxyl (−OH) group, C=C-OH. The keto form predominates at equilibrium for most ketones. Nonetheless, the enol form is important for some reactions. Furthermore, the deprotonated intermediate in the interconversion of the two forms, referred to as an enolate anion, is important in carbonyl chemistry, in large part because it is a strong nucleophile.
Mechanism
The conversion of an acid catalyzed enol to the keto form proceeds by a two step mechanism in an aqueous acidic solution.
First, the exposed electrons of the C=C double bond of the enol are donates to a hydronium ion (H3O+). This addition follows Markovnikov's rule, thus the proton is added to the carbon with more hydrogens. This is a concerted step with the oxygen in the hydroxyl group donating electrons to produce the eventual carbonyl group.
Second, the oxygen in a water molecule donates electrons to the hydrogen in the hydroxyl group, thus relieving the positive charge on the electronegative oxygen atom.
Erlenmeyer rule
One of the early investigators into keto-enol tatomerism was Richard August Carl Emil Erlenmeyer and his Erlenmeyer rule (developed in 1880) states that all alcohols in which the hydroxyl group is attached directly to a double-bonded carbon atom become aldehydes or ketones. This occurs because the keto form is generally more stable than its enol tautomer. As the lower energy form, the keto form is favored at equilibrium.
Significance in biochemistry
Keto-enol tautomerism is important in several areas of biochemistry. The high phosphate-transfer potential of phosphoenolpyruvate results from the fact that the phosphorylated compound is "trapped" in the less stable enol form, whereas after dephosphorylation it can assume the keto form. Rare enol tautomers of the bases guanine and thymine can lead to mutation because of their altered base-pairing properties.
In certain aromatic compounds such as phenols the enol is important due to the aromatic character of the enol but not the keto form. Melting the naphthalene derivative 1,4-dihydroxynaphthalene 1 at 200 °C results in a 2:1 mixture with the keto form 2. Heating the keto form in benzene at 120°C for three days also affords a mixture (1:1 with first order reaction kinetics) The keto product is kinetically stable and reverts back to the enol in presence of a base. The keto form can be obtained in a pure form by stirring the keto form in triflic acid and toluene (1:9 ratio) followed recrystallization from isopropyl ether [1].
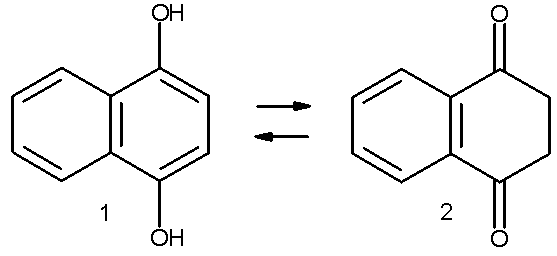
When the enol form is complexed with chromium tricarbonyl, complete conversion to the keto form accelerated and occurs even at room temperature in benzene.
DNA
In deoxyribonucleic acids(DNA), the nucleotide bases are in keto form. However, James Watson and Francis Crick first believed them to be in the enol tautomeric form, delaying the solution of the structure for several months [2].
Hydration of alkynes
Hydration of alkynes (of the general form RC≡CR, where R is an alkyl group or hydrogen), produces an enol that is in equilibrium with the keto form. If R, R', or both are hydrogen atoms, the keto form is an aldehyde. If both R and R' are alkyl groups, the keto form is a ketone. The most commonly used set of reagents is sulfuric acid (H2SO4) and mercury(II) sulfate (HgSO4).
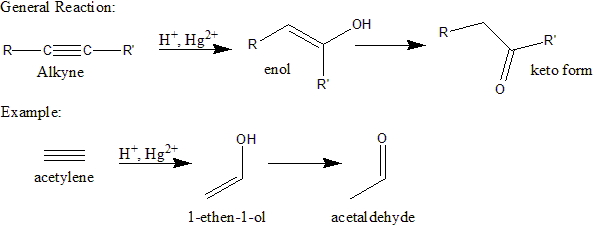
Here, acetylene (ethyne) is reacted with H2SO4 and HgSO4, adding H to one carbon and OH to the other; this forms the intermediate enol. In this reaction, the carbons are equivalent and there is no stereoselectivity. The reaction immediately continues with keto-enol tautomerization.
In general, the equilibrium lies far toward the keto side; in fact, the enol intermediate cannot be isolated as a product. Hydration of alkynes is unlike hydration of alkenes, where the product is an alcohol rather than an enol; therefore, no such equilibrium occurs.
References
- ↑ Rediscovery, Isolation, and Asymmetric Reduction of 1,2,3,4-Tetrahydronaphthalene-1,4-dione and Studies of Its [Cr(CO)3] Complex E. Peter Kündig, Alvaro Enríquez García, Thierry Lomberget, Gérald Bernardinelli Angewandte Chemie International Edition Volume 45, Issue 1 , Pages 98 - 101 2006 Abstract
- ↑ The Eighth Day of Creation. Judson, Horace Freeland. Simon & Schuster, NY:1979.