Hereditary nonpolyposis colorectal cancer pathophysiology
|
Hereditary Nonpolyposis Colorectal Cancer Microchapters |
|
Differentiating Hereditary Nonpolyposis Colorectal Cancer from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Hereditary nonpolyposis colorectal cancer pathophysiology On the Web |
|
American Roentgen Ray Society Images of Hereditary nonpolyposis colorectal cancer pathophysiology |
|
FDA on Hereditary nonpolyposis colorectal cancer pathophysiology |
|
CDC on Hereditary nonpolyposis colorectal cancer pathophysiology |
|
Hereditary nonpolyposis colorectal cancer pathophysiology in the news |
|
Blogs on Hereditary nonpolyposis colorectal cancer pathophysiology |
|
Directions to Hospitals Treating Hereditary nonpolyposis colorectal cancer |
|
Risk calculators and risk factors for Hereditary nonpolyposis colorectal cancer pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Characteristics of HNPCC-associated colon cancers
In the United States, about 160,000 new cases of colorectal cancer are diagnosed each year. Hereditary nonpolyposis colorectal cancer is responsible for approximately 2 percent to 7 percent of all diagnosed cases of colorectal cancer. The average age of diagnosis of cancer in patients with this syndrome is 44 years old, as compared to 64 years old in people without the syndrome. [1]
HNPCC defects in DNA mismatch repair lead to microsatellite instability, also known as MSI-H, which is a hallmark of HNPCC. Three major groups of MSI-H cancers can be recognized by histopathological criteria:
- (1) right-sided poorly differentiated cancers
- (2) right-sided mucinous cancers
- (3) adenocarcinomas in any location showing any measurable level of intraepithelial lymphocyte (TIL)
MSI is identifiable in cancer specimens in the pathology laboratory. [2]
Malignant consequences of the Lynch syndrome
Individuals with HNPCC have about an 80% lifetime risk for colon cancer. Two-thirds of these cancers occur in the proximal colon. The mean age of colorectal cancer diagnosis is 44 for members of families that meet the Amsterdam criteria. Also, women with HNPCC have a 30-50% lifetime risk of endometrial cancer. The average age of diagnosis of endometrial cancer is about 46 years. Among women with HNPCC who have both colon and endometrial cancer, about half present first with endometrial cancer. In HNPCC, the mean age of diagnosis of gastric cancer is 56 years of age with intestinal-type adenocarcinoma being the most commonly reported pathology. HNPCC-associated ovarian cancers have an average age of diagnosis of 42.5 years-old; approximately 30% are diagnosed before age 40 years. Other HNPCC-related cancers have been reported with specific features: the urinary tract cancers are transitional carcinoma of the ureter and renal pelvis; small bowel cancers occur most commonly in the duodenum and jejunum; the central nervous system tumor most often seen is glioblastoma.
Genetic basis of Lynch syndrome
HNPCC is known to be associated with mutations in genes involved in the DNA mismatch repair pathway
| Genes implicated in HNPCC | Frequency of mutations in HNPCC families | First publication
. |
|---|---|---|
| MLH1 | Together with MSH2, 90% of mutations in HNPCC families. The human mutL homologue hMLH1 is located at chromosome 3p21 | Papadopoulos et al., 1994[3] |
| MSH2 | Together with MLH1, 90% of mutations in HNPCC families.hMSH2 is gene is located at chromosome 2p21. | Fishel et al., 1993[4] |
| MSH6 | 7-10% of mutations in HNPCC families | |
| PMS | <5% of mutations in HNPCC families | |
| PMS2 | <5% of mutations in HNPCC families |
Up to 39% of families with mutations in an HNPCC gene do not meet the Amsterdam criteria. Therefore, families found to have a deleterious mutation in an HNPCC gene should be considered to have HNPCC regardless of the extent of the family history. This also means that the Amsterdam criteria fail to identify many patients at risk for Lynch syndrome. Improving the criteria for screening is an active area of research, as detailed in the Screening Strategies section of this article.
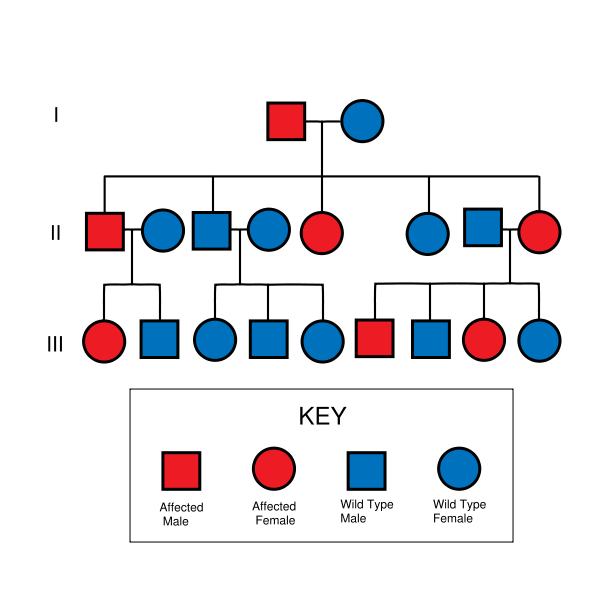
HNPCC is inherited in an autosomal dominant manner. Most people with HNPCC inherit the condition from a parent. However, due to incomplete penetrance, variable age of cancer diagnosis, cancer risk reduction, or early death, not all patients with an HNPCC gene mutation have a parent who had cancer. Some patients develop HNPCC de-novo in a new generation, without inheriting the gene. These patients are often only identified after developing an early-life colon cancer. Parents with HNPCC have a 50% chance to pass the gene on to each child.
References
- ↑ http://www.oncolink.org/types/article.cfm?c=5&s=11&ss=81&id=6979
- ↑ http://www.annalsnyas.org/cgi/content/abstract/910/1/62
- ↑ Papadopoulos N, Nicolaides N, Wei Y, Ruben S, Carter K, Rosen C, Haseltine W, Fleischmann R, Fraser C, Adams M (1994). "Mutation of a mutL homolog in hereditary colon cancer". Science. 263 (5153): 1625–9. PMID 8128251.
- ↑ Fishel R, Lescoe M, Rao M, Copeland N, Jenkins N, Garber J, Kane M, Kolodner R (1993). "The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer". Cell. 75 (5): 1027–38. PMID 8252616.