Chikungunya other diagnostic studies: Difference between revisions
No edit summary |
m (Changes made per Mahshid's request) |
||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 2: | Line 2: | ||
{{Chikungunya}} | {{Chikungunya}} | ||
{{CMG}}; {{AE}} {{AL}}, {{VR}} | |||
==Overview== | |||
Laboratory diagnosis can be accomplished by testing serum or plasma to detect [[virus]], [[viral]] nucleic acid, or virus-specific [[immunoglobulin]] (Ig) M and neutralizing [[antibodies]]. [[Viral culture]] may detect [[virus]] in the first 3 days of illness; however, Chikungunya [[virus]] should be handled under [[biosafety level|biosafety level (BSL) 3]] conditions. During the first 8 days of illness, Chikungunya [[viral]] [[RNA]] can often be identified in [[serum]]. Chikungunya [[virus]] [[antibodies]] normally develop toward the end of the first week of illness. Therefore, to definitively rule out the diagnosis, convalescent-phase samples should be obtained from patients whose acute-phase samples test negative. | |||
Chikungunya [[virus]] testing is performed at CDC, a few state health departments, and one commercial laboratory. Test results are normally available 4 to 14 days after specimen receipt. Reporting times for test results may be longer during summer months when [[arbovirus]] activity increases. Receipt of a hard copy of the results will take at least 2 weeks after testing is completed. Initial [[serological testing]] will be performed using IgM-capture [[ELISA]] and IgG [[ELISA]]. If the initial results are positive, further confirmatory testing will be performed and it may delay the reporting of final results. | |||
==Other Diagnostic Studies <SMALL><SMALL><SMALL><SMALL><SMALL>Adapted from ''Preparedness and Response for Chikungunya Virus: Introduction in the Americas. PAHO © 2011.''<ref name=PAHO2011>{{cite book | last = | first = | title = Preparedness and response for Chikungunya virus introduction in the Americas | publisher = Pan American Health Organization CDC, Center for Disease Control and Prevention | location = Washington, DC | year = 2011 | isbn = 978-92-75-11632-6 }}</ref></SMALL></SMALL></SMALL></SMALL></SMALL>== | |||
Samples collected during the first week after onset of symptoms should be tested by both [[serology|serological]] ([[IgM]] and [[IgG]] [[ELISA]]) and [[virology|virological]] ([[RT-PCR]] and isolation) methods. Specimens are usually [[blood]] or [[serum]], but in neurological cases with [[meningoencephalitis|meningoencephalitic]] features, [[CSF|cerebrospinal fluid (CSF)]] may also be obtained. Limited information is available for the detection of virus by isolation or [[RT-PCR]] from tissues or organs. In suspected fatal cases, virus detection can be attempted on available specimens. Selection of the appropriate laboratory test is based upon the source of the specimen (human or field-collected [[mosquito]]es) and the time of sample collection relative to symptom onset for humans. | |||
Three main types of laboratory tests are used for diagnosing Chikungunya virus (CHIKV): '''[[Primary isolate|virus isolation]]''', '''[[RT-PCR|reverse transcriptase-polymerase chain reaction (RT-PCR)]]''', and '''[[serology]]'''. | |||
===Virus Isolation=== | |||
Virus isolation can be performed on field collected [[mosquito]]es or acute [[serum]] specimens (≤8 days). [[Serum]] obtained from [[whole blood]] collected during the first week of illness and transported cold (between 2°–8°C or dry ice) as soon as possible (within 48 hours) to the laboratory can be inoculated into a susceptible cell line or suckling mouse. [[CHIKV]] will produce typical [[cytopathic effect|cytopathic effects (CPE)]] within three days after inoculation in a variety of [[cell line]]s, including Vero, BHK- 21, and [[HeLa]] cells. Virus isolation can be performed in T-25 flasks or shell vials. Recent data suggest that isolation in shell vials is both more sensitive and produces [[CPE]] earlier than conventional isolation in flasks. [[CHIKV]] isolation must be confirmed either by [[immunofluorescence assay|immunofluorescence assay (IFA)]], using [[CHIKV]]-specific [[antiserum]], or by [[RT-PCR]] of the culture supernatant or mouse brain suspension. Virus isolation must only be carried out in [[biosafety level#The Levels|biosafety level 3 (BSL-3)]] laboratories to reduce the risk of viral transmission. | |||
===RT-PCR=== | |||
Several [[RT-PCR]] assays for the detection of [[CHIKV]] [[RNA]] have been published. Real time, closed system assays should be utilized, due to their increased [[sensitivity]] and lower risk of [[contamination]]. The Arboviral Diagnostic Laboratory within the Division of Vector-Borne Diseases (DVBD) routinely utilizes the published assay, which demonstrates a [[sensitivity]] of less than 1 pfu or 50 [[genome]] copies. [[Serum]] from whole blood is used for [[PCR]] testing as well as [[primary isolate|virus isolation]].<ref name="LanciottiKosoy2007">{{cite journal|last1=Lanciotti|first1=Robert S.|last2=Kosoy|first2=Olga L.|last3=Laven|first3=Janeen J.|last4=Panella|first4=Amanda J.|last5=Velez|first5=Jason O.|last6=Lambert|first6=Amy J.|last7=Campbell|first7=Grant L.|title=Chikungunya Virus in US Travelers Returning from India, 2006|journal=Emerging Infectious Diseases|volume=13|issue=5|year=2007|pages=764–767|issn=1080-6040|doi=10.3201/eid1305.070015}}</ref> | |||
===Serological Tests=== | |||
For [[serology|serological]] diagnosis, serum obtained from [[whole blood]] is utilized in [[ELISA|enzyme-linked immunosorbent assay (ELISA)]] and [[PRNT|plaque reduction neutralization testing (PRNT)]]. The [[serum]] (or [[blood]]) specimen should be transported at 2°–8°C and should not be frozen. Serologic diagnosis can be made by demonstration of [[IgM]] antibodies specific for [[CHIKV]] or by a four-fold rise in [[PRNT]] titer in acute and convalescent specimens. [[IgM]] antibodies specific for [[CHIKV]] are demonstrated by using the [[IgM]] antibody capture [[ELISA]] (MAC-ELISA), followed by the [[PRNT]]. As of 2010, there were no World Health Organization (WHO) validated commercial [[IgM]] [[ELISA]]s available. [[PRNT]] is required to confirm the MAC-[[ELISA]] results, since cross-reactivity in the MAC-[[ELISA]] between some members of the Semliki Forest virus (SFV) serogroup has been observed. [[PRNT]] testing, whether used to confirm the MAC-[[ELISA]] or to demonstrate a four-fold rise in acute/convalescent specimens, should always include other viruses within the SFV serogroup (e.g., Mayaro virus) to validate specificity of reactivity. In situations where the PRNT assay is not available, other serological tests (e.g. [[hemagglutination inhibition assay|hemagglutination inhibition assay]]) can be used to identify a recent [[alphavirus]] infection; however, [[PRNT]] is required to confirm a recent [[CHIKV]] infection. | |||
An acute phase [[serum]] should be collected immediately after the onset of illness and the convalescent phase [[serum]] 10–14 days later. [[CHIKV]]-specific [[IgM]] and neutralizing antibodies normally develop towards the end of the first week of illness. Therefore, to definitively rule out the diagnosis, convalescent samples should be obtained on patients whose acute samples test negative. | |||
==Collection, Storage, and Transportation of Samples== | |||
===Collection of Samples for Serology, Isolation, and Molecular Diagnosis:=== | |||
* Sample: | |||
:* [[Serum]] | |||
* Time of collection: | |||
:* Acute — within the first eight days of illness | |||
:* Convalescent — 10–14 days after acute specimen collection | |||
* To collect [[serum]]: | |||
:* Aseptically collect 4–5 ml of venous blood in a tube or a vial. | |||
:* Allow blood to [[clot]] at room [[temperature]], centrifuge at 2,000 rpm to separate serum. Collect the serum in a clean dry vial. | |||
:* All clinical samples should be accompanied by their clinical and epidemiological information. | |||
===Other Types of Specimens for Laboratory Investigation:=== | |||
* Specimens: | |||
:* [[CSF]] in [[meningoencephalitis]] cases. | |||
:* [[Synovial fluid]] in [[arthritis]] with effusion. | |||
:* [[Autopsy]] material – [[serum]] or available tissues. (Note: [[Mosquito]]es collected in the field will also be handled using the same techniques described here.) | |||
* Transportation of Samples: | |||
:* Transport specimens to the laboratory at 2°−8°C (icebox) as soon as possible. | |||
:* Do not freeze [[whole blood]], as [[hemolysis]] may interfere with [[serology]] test results. | |||
:* If a delay greater than 24 hours is expected before specimens can be submitted to the laboratory, the [[serum]] should be separated and stored at refrigerated temperature. | |||
:* Serum samples for [[primary isolate|virus isolation]] and molecular diagnosis should be stored frozen (at –20°C for short-term storage or at –70°C for long-term storage). | |||
==Laboratory Surveillance== | |||
Prior to identification of [[CHIKV]] in a country, laboratory surveillance should be conducted on three sets of samples, as follows: | |||
* Dengue-negative specimens where the patient exhibits severe [[joint pain]] | |||
* Samples with clinically compatible illness from new geographic areas without active [[dengue]] circulation | |||
* Clusters of [[febrile]] illness with severe [[joint pain]] | |||
The following table outlines the ideal tests to be performed in various epidemiological settings. | |||
{| style="border: 0px; font-size: 85%; margin: 3px;" align=center | |||
! style="background: #4479BA; width: 25%;" | {{fontcolor|#FFF|Epidemiological scenario}} | |||
! style="background: #4479BA; width: 25%;" | {{fontcolor|#FFF|Testing to be performed}} | |||
! style="background: #4479BA; width: 50%;" | {{fontcolor|#FFF|Samples to test}} | |||
|- | |||
! style="padding: 5px 5px; background: #DCDCDC;" | No signs of transmission | |||
| style="padding: 5px 5px; background: #DCDCDC;" | IgM ELISA, IgG ELISA | |||
| style="padding: 5px 5px; background: #DCDCDC;" | All samples from patients exhibiting clinically compatible illness | |||
|- | |||
! style="padding: 5px 5px; background: #F5F5F5;" | Suspect CHIKV illness | |||
| style="padding: 5px 5px; background: #F5F5F5;" | IgM ELISA, IgG ELISA, real-time RT-PCR, virus isolation, PRNT | |||
| style="padding: 5px 5px; background: #F5F5F5;" | All samples from patients exhibiting clinically compatible illness | |||
|- | |||
! style="padding: 5px 5px; background: #DCDCDC;" | Continued transmission | |||
| style="padding: 5px 5px; background: #DCDCDC;" | IgM ELISA, IgG ELISA, real-time RT-PCR; limited virus isolation | |||
| style="padding: 5px 5px; background: #DCDCDC;" | Subset samples from classical CHIK cases, as determined by lab constraints and epidemiological status; Samples from all atypical or severe cases should be tested | |||
|- | |||
! style="padding: 5px 5px; background: #F5F5F5;" | Periodic outbreaks or active surveillance in areas near CHIKV transmission | |||
| style="padding: 5px 5px; background: #F5F5F5;" | IgM ELISA, IgG ELISA, real-time RT-PCR; limited virus isolation | |||
| style="padding: 5px 5px; background: #F5F5F5;" | Subset of samples from classical CHIK cases, as determined by lab constraints and epidemiological status; samples from all atypical or severe cases should be tested | |||
|} | |||
During the initial introduction of [[CHIKV]] into a new region, comprehensive testing should be completed to confirm that [[CHIKV]] is the etiological agent. After CHIKV has been identified, limited testing (not testing all specimens or performing fewer assay types) can be considered depending upon the capacity of the lab and the epidemiological situation. | |||
==Interpretation and Reporting of Results== | |||
{| style="float: right;" | |||
| valign=top | [[File:Viremia and immune response following Chikungunya virus infection.jpg|375px|thumb|none|Courtesy: Center for Disease Control and Prevention<ref name=PAHO2011>{{cite book | last = | first = | title = Preparedness and response for Chikungunya virus introduction in the Americas | publisher = PAHO, Pan American Health Organization; CDC, Center for Disease Control and Prevention | location = Washington, DC | year = 2011 | isbn = 978-92-75-11632-6 }}</ref>]] | |||
|- | |||
| valign=top | | |||
{| style="border: 0px; font-size: 80%; margin: 3px;" align=center | |||
|+ '''Typical results of samples tested at various time points''' | |||
! style="background: #4479BA; width: 125px;" | {{fontcolor|#FFF|Days post illness onset}} | |||
! style="background: #4479BA; width: 125px;" | {{fontcolor|#FFF|Virus testing}} | |||
! style="background: #4479BA; width: 125px;" | {{fontcolor|#FFF|Antibody testing}} | |||
|- | |||
! style="padding: 5px 5px; background: #DCDCDC;" | Day 1–3 | |||
! style="padding: 5px 5px; background: #DCDCDC;" | RT-PCR = Positive <BR> Isolation = Positive | |||
! style="padding: 5px 5px; background: #DCDCDC;" | IgM = Negative <BR> PRNT = Negative | |||
|- | |||
! style="padding: 5px 5px; background: #DCDCDC;" | Day 4–8 | |||
! style="padding: 5px 5px; background: #DCDCDC;" | RT-PCR = Positive <BR> Isolation = Negative | |||
! style="padding: 5px 5px; background: #DCDCDC;" | IgM = Positive <BR> PRNT = Negative | |||
|- | |||
! style="padding: 5px 5px; background: #DCDCDC;" | >Day 8 | |||
! style="padding: 5px 5px; background: #DCDCDC;" | RT-PCR = Negative <BR> Isolation = Negative | |||
! style="padding: 5px 5px; background: #DCDCDC;" | IgM = Positive <BR> PRNT = Positive | |||
|} | |||
|} | |||
The figure shows typical viremia and antibody response in humans following Chikungunya virus infection and the table describes the typical results of testing samples at various time points. | |||
The following laboratory test results would confirm a recent CHIKV infection: | |||
* Isolation of [[CHIKV]], including confirmatory identification (either [[Immunofluorescence|IFA]], [[RT-PCR]], or [[sequencing]]). | |||
* Detection of [[CHIKV]] [[RNA]] by real time [[RT-PCR]]. | |||
* Identification of a positive [[IgM]] result in a patient with acute symptoms of [[CHIKV]], followed by the demonstration of CHIKV-specific antibody determined by [[PRNT]] with viruses in the SFV serogroup. | |||
* Demonstration of [[seroconversion]] or a four-fold rise in [[PRNT]], [[HIA|HI]], or [[ELISA]] titers (again using other SFV serogroup viruses) between acute and convalescent specimens. | |||
Autochthonous cases should be reported to WHO, in collaboration with an epidemiologist, according to International Health Regulations (IHR). | |||
==References== | ==References== | ||
{{ | |||
{{reflist|2}} | |||
==External Links== | |||
* [http://www.cdc.gov/Chikungunya/index.html CDC Chikungunya virus] | |||
[[Category:Disease]] | [[Category:Disease]] | ||
[[Category:Viral diseases]] | [[Category:Viral diseases]] | ||
[[Category:Togaviruses]] | [[Category:Togaviruses]] | ||
[[Category:Tropical disease]] | [[Category:Tropical disease]] | ||
Latest revision as of 17:23, 18 September 2017
|
Chikungunya Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Chikungunya other diagnostic studies On the Web |
|
American Roentgen Ray Society Images of Chikungunya other diagnostic studies |
|
Risk calculators and risk factors for Chikungunya other diagnostic studies |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alejandro Lemor, M.D. [2], Vendhan Ramanujam M.B.B.S [3]
Overview
Laboratory diagnosis can be accomplished by testing serum or plasma to detect virus, viral nucleic acid, or virus-specific immunoglobulin (Ig) M and neutralizing antibodies. Viral culture may detect virus in the first 3 days of illness; however, Chikungunya virus should be handled under biosafety level (BSL) 3 conditions. During the first 8 days of illness, Chikungunya viral RNA can often be identified in serum. Chikungunya virus antibodies normally develop toward the end of the first week of illness. Therefore, to definitively rule out the diagnosis, convalescent-phase samples should be obtained from patients whose acute-phase samples test negative.
Chikungunya virus testing is performed at CDC, a few state health departments, and one commercial laboratory. Test results are normally available 4 to 14 days after specimen receipt. Reporting times for test results may be longer during summer months when arbovirus activity increases. Receipt of a hard copy of the results will take at least 2 weeks after testing is completed. Initial serological testing will be performed using IgM-capture ELISA and IgG ELISA. If the initial results are positive, further confirmatory testing will be performed and it may delay the reporting of final results.
Other Diagnostic Studies Adapted from Preparedness and Response for Chikungunya Virus: Introduction in the Americas. PAHO © 2011.[1]
Samples collected during the first week after onset of symptoms should be tested by both serological (IgM and IgG ELISA) and virological (RT-PCR and isolation) methods. Specimens are usually blood or serum, but in neurological cases with meningoencephalitic features, cerebrospinal fluid (CSF) may also be obtained. Limited information is available for the detection of virus by isolation or RT-PCR from tissues or organs. In suspected fatal cases, virus detection can be attempted on available specimens. Selection of the appropriate laboratory test is based upon the source of the specimen (human or field-collected mosquitoes) and the time of sample collection relative to symptom onset for humans.
Three main types of laboratory tests are used for diagnosing Chikungunya virus (CHIKV): virus isolation, reverse transcriptase-polymerase chain reaction (RT-PCR), and serology.
Virus Isolation
Virus isolation can be performed on field collected mosquitoes or acute serum specimens (≤8 days). Serum obtained from whole blood collected during the first week of illness and transported cold (between 2°–8°C or dry ice) as soon as possible (within 48 hours) to the laboratory can be inoculated into a susceptible cell line or suckling mouse. CHIKV will produce typical cytopathic effects (CPE) within three days after inoculation in a variety of cell lines, including Vero, BHK- 21, and HeLa cells. Virus isolation can be performed in T-25 flasks or shell vials. Recent data suggest that isolation in shell vials is both more sensitive and produces CPE earlier than conventional isolation in flasks. CHIKV isolation must be confirmed either by immunofluorescence assay (IFA), using CHIKV-specific antiserum, or by RT-PCR of the culture supernatant or mouse brain suspension. Virus isolation must only be carried out in biosafety level 3 (BSL-3) laboratories to reduce the risk of viral transmission.
RT-PCR
Several RT-PCR assays for the detection of CHIKV RNA have been published. Real time, closed system assays should be utilized, due to their increased sensitivity and lower risk of contamination. The Arboviral Diagnostic Laboratory within the Division of Vector-Borne Diseases (DVBD) routinely utilizes the published assay, which demonstrates a sensitivity of less than 1 pfu or 50 genome copies. Serum from whole blood is used for PCR testing as well as virus isolation.[2]
Serological Tests
For serological diagnosis, serum obtained from whole blood is utilized in enzyme-linked immunosorbent assay (ELISA) and plaque reduction neutralization testing (PRNT). The serum (or blood) specimen should be transported at 2°–8°C and should not be frozen. Serologic diagnosis can be made by demonstration of IgM antibodies specific for CHIKV or by a four-fold rise in PRNT titer in acute and convalescent specimens. IgM antibodies specific for CHIKV are demonstrated by using the IgM antibody capture ELISA (MAC-ELISA), followed by the PRNT. As of 2010, there were no World Health Organization (WHO) validated commercial IgM ELISAs available. PRNT is required to confirm the MAC-ELISA results, since cross-reactivity in the MAC-ELISA between some members of the Semliki Forest virus (SFV) serogroup has been observed. PRNT testing, whether used to confirm the MAC-ELISA or to demonstrate a four-fold rise in acute/convalescent specimens, should always include other viruses within the SFV serogroup (e.g., Mayaro virus) to validate specificity of reactivity. In situations where the PRNT assay is not available, other serological tests (e.g. hemagglutination inhibition assay) can be used to identify a recent alphavirus infection; however, PRNT is required to confirm a recent CHIKV infection.
An acute phase serum should be collected immediately after the onset of illness and the convalescent phase serum 10–14 days later. CHIKV-specific IgM and neutralizing antibodies normally develop towards the end of the first week of illness. Therefore, to definitively rule out the diagnosis, convalescent samples should be obtained on patients whose acute samples test negative.
Collection, Storage, and Transportation of Samples
Collection of Samples for Serology, Isolation, and Molecular Diagnosis:
- Sample:
- Time of collection:
- Acute — within the first eight days of illness
- Convalescent — 10–14 days after acute specimen collection
- To collect serum:
- Aseptically collect 4–5 ml of venous blood in a tube or a vial.
- Allow blood to clot at room temperature, centrifuge at 2,000 rpm to separate serum. Collect the serum in a clean dry vial.
- All clinical samples should be accompanied by their clinical and epidemiological information.
Other Types of Specimens for Laboratory Investigation:
- Specimens:
- CSF in meningoencephalitis cases.
- Synovial fluid in arthritis with effusion.
- Autopsy material – serum or available tissues. (Note: Mosquitoes collected in the field will also be handled using the same techniques described here.)
- Transportation of Samples:
- Transport specimens to the laboratory at 2°−8°C (icebox) as soon as possible.
- Do not freeze whole blood, as hemolysis may interfere with serology test results.
- If a delay greater than 24 hours is expected before specimens can be submitted to the laboratory, the serum should be separated and stored at refrigerated temperature.
- Serum samples for virus isolation and molecular diagnosis should be stored frozen (at –20°C for short-term storage or at –70°C for long-term storage).
Laboratory Surveillance
Prior to identification of CHIKV in a country, laboratory surveillance should be conducted on three sets of samples, as follows:
- Dengue-negative specimens where the patient exhibits severe joint pain
- Samples with clinically compatible illness from new geographic areas without active dengue circulation
- Clusters of febrile illness with severe joint pain
The following table outlines the ideal tests to be performed in various epidemiological settings.
| Epidemiological scenario | Testing to be performed | Samples to test |
|---|---|---|
| No signs of transmission | IgM ELISA, IgG ELISA | All samples from patients exhibiting clinically compatible illness |
| Suspect CHIKV illness | IgM ELISA, IgG ELISA, real-time RT-PCR, virus isolation, PRNT | All samples from patients exhibiting clinically compatible illness |
| Continued transmission | IgM ELISA, IgG ELISA, real-time RT-PCR; limited virus isolation | Subset samples from classical CHIK cases, as determined by lab constraints and epidemiological status; Samples from all atypical or severe cases should be tested |
| Periodic outbreaks or active surveillance in areas near CHIKV transmission | IgM ELISA, IgG ELISA, real-time RT-PCR; limited virus isolation | Subset of samples from classical CHIK cases, as determined by lab constraints and epidemiological status; samples from all atypical or severe cases should be tested |
During the initial introduction of CHIKV into a new region, comprehensive testing should be completed to confirm that CHIKV is the etiological agent. After CHIKV has been identified, limited testing (not testing all specimens or performing fewer assay types) can be considered depending upon the capacity of the lab and the epidemiological situation.
Interpretation and Reporting of Results
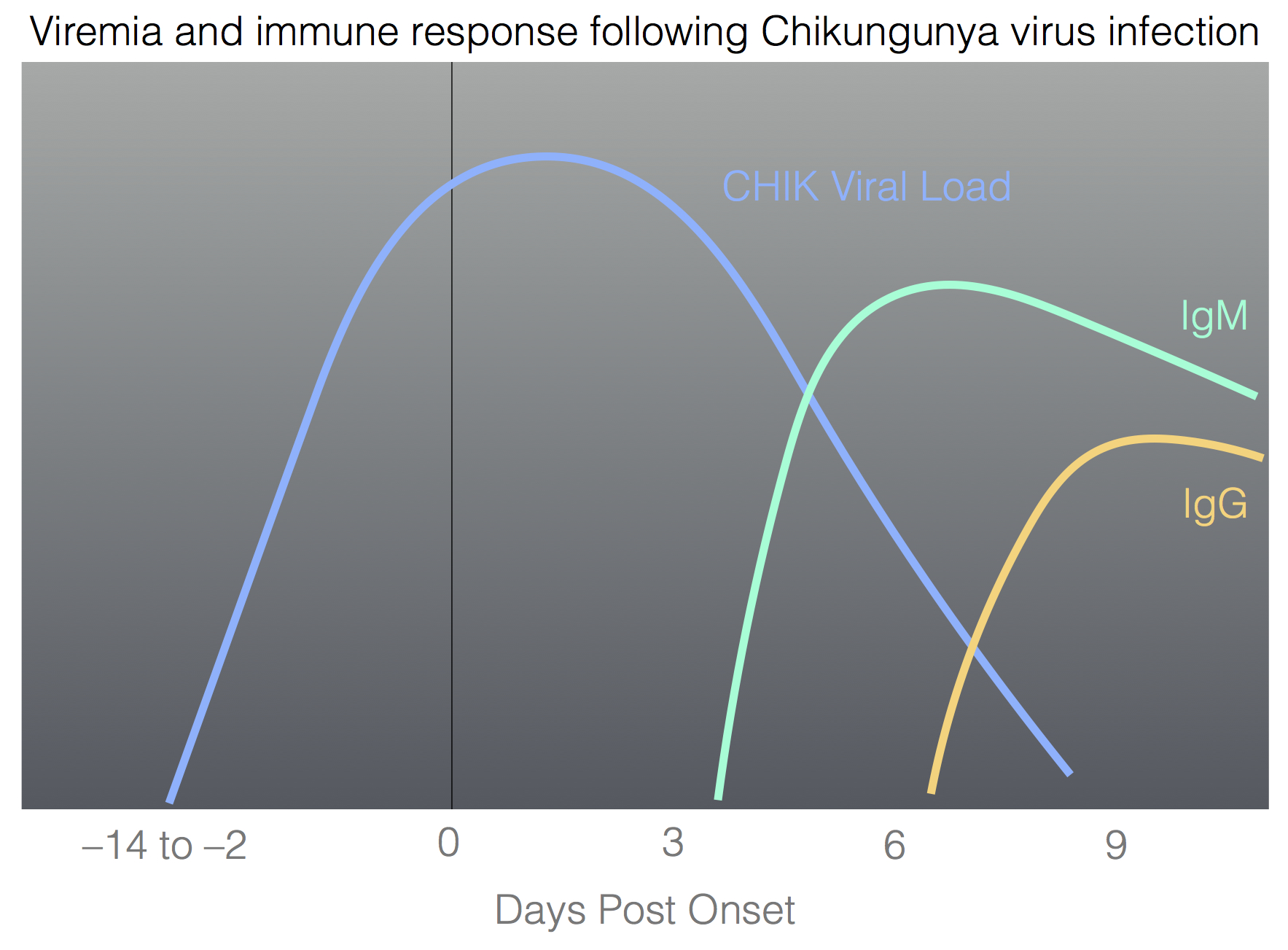 | ||||||||||||
|
The figure shows typical viremia and antibody response in humans following Chikungunya virus infection and the table describes the typical results of testing samples at various time points.
The following laboratory test results would confirm a recent CHIKV infection:
- Isolation of CHIKV, including confirmatory identification (either IFA, RT-PCR, or sequencing).
- Detection of CHIKV RNA by real time RT-PCR.
- Identification of a positive IgM result in a patient with acute symptoms of CHIKV, followed by the demonstration of CHIKV-specific antibody determined by PRNT with viruses in the SFV serogroup.
- Demonstration of seroconversion or a four-fold rise in PRNT, HI, or ELISA titers (again using other SFV serogroup viruses) between acute and convalescent specimens.
Autochthonous cases should be reported to WHO, in collaboration with an epidemiologist, according to International Health Regulations (IHR).
References
- ↑ 1.0 1.1 Preparedness and response for Chikungunya virus introduction in the Americas. Washington, DC: Pan American Health Organization CDC, Center for Disease Control and Prevention. 2011. ISBN 978-92-75-11632-6.
- ↑ Lanciotti, Robert S.; Kosoy, Olga L.; Laven, Janeen J.; Panella, Amanda J.; Velez, Jason O.; Lambert, Amy J.; Campbell, Grant L. (2007). "Chikungunya Virus in US Travelers Returning from India, 2006". Emerging Infectious Diseases. 13 (5): 764–767. doi:10.3201/eid1305.070015. ISSN 1080-6040.