Aripiprazole (intramuscular): Difference between revisions
No edit summary |
Martin Nino (talk | contribs) No edit summary |
||
| (10 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{ | |authorTag={{MN}} | ||
|genericName=Aripiprazole | |genericName=Aripiprazole lauroxil | ||
|aOrAn=an | |aOrAn=an | ||
|drugClass=atypical antipsychotic | |drugClass=atypical [[antipsychotic]] | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=[[schizophrenia]] | |indication=patients with [[schizophrenia]] | ||
|adverseReactions=akathisia (≥5%) | |||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
| | |blackBoxWarningTitle=<span style="color:#FF0000;">WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS</span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;"> | |||
|blackBoxWarningBody= | Elderly patients with [[dementia]]-related [[psychosis]] treated with antipsychotic drugs are at an increased risk of death. Aripiprazole lauroxil is not approved for the treatment of patients with dementia-related psychosis. | ||
</span></i> | |||
|fdaLIADAdult= | |||
======Indications====== | |||
Aripiprazole lauroxil is indicated for treatment of [[schizophrenia]]. | |||
* | ======Dosage====== | ||
:*'''Treatment of [[Schizophrenia]]''' | |||
::*Aripiprazole lauroxil is only to be administered as an [[intramuscular]] injection by a healthcare professional. For patients who have never taken aripiprazole, establish tolerability with oral aripiprazole prior to initiating treatment with Aripiprazole lauroxil. Due to the [[half-life]] of oral aripiprazole, it may take up to 2 weeks to fully assess tolerability. Refer to the prescribing information of oral aripiprazole for the recommended dosage and administration of the oral formulation. | |||
* | ::*Depending on individual patient's needs, treatment with Aripiprazole lauroxil can be initiated at a dose of 441 mg, 662 mg or 882 mg administered monthly, which corresponds to 300 mg, 450 mg and 600 mg of aripiprazole, respectively. Treatment may also be initiated with the 882 mg dose every 6 weeks. | ||
:* | ::*Administer Aripiprazole lauroxil either in the deltoid muscle (441 mg dose only) or gluteal muscle (441 mg, 662 mg or 882 mg). | ||
:* | :*'''Table 1: Aripiprazole lauroxil Dosing Frequency and Site of Injection''' | ||
[[File:table1_ari.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
::*Use the following Aripiprazole lauroxil doses for patients who are stabilized on oral aripiprazole, as shown in TABLE 2. | |||
* | :*'''Table 2: Aripiprazole lauroxil Doses Based on Oral Aripiprazole Total Daily Dose''' | ||
[[File:table2_ari.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
::*In conjunction with the first Aripiprazole lauroxil injection, administer treatment with oral aripiprazole for 21 consecutive days. | |||
::* | |||
::*Dose may be adjusted as needed. When making dose and dosing interval adjustments, the [[pharmacokinetics]] and prolonged-release characteristics of Aripiprazole lauroxil should be considered. | |||
::* | |||
:*'''Missed Doses''' | |||
::*When a dose is missed, administer the next injection of Aripiprazole lauroxil as soon as possible. If the time elapsed since the last Aripiprazole lauroxil injection exceeds the length of time noted in TABLE 3, use oral aripiprazole supplementation with the next Aripiprazole lauroxil injection as recommended below. | |||
* | :*'''Table 3: Recommendation for Concomitant Oral Aripiprazole Supplementation Following Missed Doses(a)''' | ||
[[File:table3_ari.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
<SMALL>ARISTADA: Aripiprazole lauroxil's Brand name</SMALL> | |||
:*'''Early Dosing''' | |||
::*The recommended Aripiprazole lauroxil dosing interval is either monthly for the 441 mg, 662 mg and 882 mg doses or every 6 weeks for 882 mg dose and should be maintained. In the event of early dosing, an Aripiprazole lauroxil injection should not be given earlier than 14 days after the previous injection. | |||
* | :*'''Dose Adjustments for [[CYP450]] Considerations''' | ||
::*Refer to the prescribing information for oral aripiprazole for recommendations regarding dosage adjustments due to drug interactions, for the first 21 days when the patient is taking oral aripiprazole concomitantly with the first dose of Aripiprazole lauroxil. | |||
:* | ::*Once stabilized on Aripiprazole lauroxil, refer to the dosing recommendations below for patients taking [[CYP2D6]] inhibitors, [[CYP3A4]] inhibitors, or [[CYP3A4]] inducers: | ||
:::- No dosage changes recommended for Aripiprazole lauroxil, if [[CYP450]] modulators are added for less than 2 weeks. | |||
:::- Make dose changes to Aripiprazole lauroxil if [[CYP450]] modulators are added for greater than 2 weeks (see TABLE 4). | |||
* | :*'''Table 4: Aripiprazole lauroxil Dose Adjustments with Concomitant CYP450 Modulator Use''' | ||
[[File:table4_ari.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
<SMALL>ARISTADA: Aripiprazole lauroxil's Brand name</SMALL> | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Aripiprazole lauroxil in adult patients. | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Aripiprazole lauroxil in adult patients. | |||
|fdaLIADPed=Safety and effectiveness of Aripiprazole lauroxil in patients <18 years of age have not been evaluated. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Aripiprazole lauroxil in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Aripiprazole lauroxil in pediatric patients. | |||
|contraindications=Aripiprazole lauroxil is contraindicated in patients with a known [[hypersensitivity]] reaction to aripiprazole. Hypersensitivity reactions have ranged from [[pruritus]]/[[urticaria]] to [[anaphylaxis]]. | |||
|warnings= | |||
======Increased Mortality in Elderly Patients with [[Dementia]]-related [[Psychosis]]====== | |||
Elderly patients with dementia-related psychosis treated with [[antipsychotic]] drugs are at an increased risk of death. Analyses of 17 [[placebo]]-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. | |||
Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., [[heart failure]], [[sudden death]]) or infectious (e.g., [[pneumonia]]) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Aripiprazole lauroxil is not approved for the treatment of patients with dementia-related psychosis. | |||
======Cerebrovascular Adverse Reactions, Including [[Stroke]]====== | |||
In [[placebo]]-controlled trials with [[risperidone]], [[aripiprazole]], and [[olanzapine]] in elderly patients with [[dementia]], there was a higher incidence of cerebrovascular adverse reactions ([[cerebrovascular accidents]] and [[transient ischemic attack]]s) including fatalities compared to placebo-treated patients. Aripiprazole lauroxil is not approved for the treatment of patients with dementia-related psychosis. | |||
======[[Neuroleptic Malignant Syndrome]]====== | |||
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) may occur in association with [[antipsychotic]] drugs, including Aripiprazole lauroxil. Clinical manifestations of NMS are [[hyperpyrexia]], [[muscle rigidity]], altered mental status, and evidence of [[autonomic instability]] (irregular pulse or blood pressure, [[tachycardia]], [[diaphoresis]], and cardiac [[dysrhythmia]]). Additional signs may include elevated [[creatine phosphokinase]], [[myoglobinuria]] ([[rhabdomyolysis]]), and [[acute renal failure]]. | |||
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases in which the clinical presentation includes both serious medical illness (e.g., [[pneumonia]], systemic infection, etc.) and untreated or inadequately treated [[extrapyramidal sign]]s and symptoms (EPS). Other important considerations in the differential diagnosis include central [[anticholinergic]] toxicity, [[heat stroke]], [[drug fever]], and primary [[central nervous system]] pathology. | |||
The management of NMS should include: (1) immediate discontinuation of [[antipsychotic]] drugs and other drugs not essential to concurrent therapy; (2) intensive symptomatic treatment and medical monitoring; and (3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS. | |||
If a patient appears to require antipsychotic drug treatment after recovery from NMS, reintroduction of drug therapy should be closely monitored, since recurrences of NMS have been reported. | |||
======[[Tardive Dyskinesia]]====== | |||
A syndrome of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with [[antipsychotic]] drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to predict which patients will develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown. | |||
The risk of developing tardive dyskinesia and the likelihood that it will become irreversible appear to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase, but the syndrome can develop after relatively brief treatment periods at low doses, although this is uncommon. | |||
There is no known treatment for established tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment itself may suppress (or partially suppress) the signs and symptoms of the syndrome and may thus mask the underlying process. The effect of symptomatic suppression on the long-term course of the syndrome is unknown. | |||
Given these considerations, Aripiprazole lauroxil should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that is known to respond to antipsychotic drugs. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically. | |||
If signs and symptoms of tardive dyskinesia appear in a patient treated with Aripiprazole lauroxil drug discontinuation should be considered. However, some patients may require treatment with Aripiprazole lauroxil despite the presence of the syndrome. | |||
======Metabolic Changes====== | |||
Atypical [[antipsychotic]] drugs have been associated with metabolic changes that include [[hyperglycemia]]/[[diabetes mellitus]], [[dyslipidemia]], and weight gain. While all drugs in the class have been shown to produce some metabolic changes, each drug has its own specific risk profile. | |||
:*'''[[Hyperglycemia]]/ [[Diabetes Mellitus]]''' | |||
* | |||
::*Hyperglycemia, in some cases extreme and associated with [[ketoacidosis]] or [[hyperosmolar coma]] or death, has been reported in patients treated with atypical [[antipsychotics]]. There have been reports of hyperglycemia in patients treated with oral aripiprazole. Assessment of the relationship between atypical antipsychotic use and [[glucose]] abnormalities is complicated by the possibility of an increased background risk of diabetes mellitus in patients with [[schizophrenia]] and the increasing incidence of diabetes mellitus in the general population. Given these confounders, the relationship between atypical antipsychotic use and hyperglycemia-related adverse events is not completely understood. However, epidemiological studies suggest an increased risk of hyperglycemia-related adverse reactions in patients treated with the atypical antipsychotics. | |||
* | |||
::*Patients with an established diagnosis of diabetes mellitus who are started on atypical antipsychotics should be monitored regularly for worsening of glucose control. Patients with risk factors for diabetes mellitus (e.g., [[obesity]], family history of diabetes) who are starting treatment with atypical antipsychotics should undergo fasting blood glucose testing at the beginning of treatment and periodically during treatment. Any patient treated with atypical antipsychotics should be monitored for symptoms of hyperglycemia including [[polydipsia]], [[polyuria]], [[polyphagia]], and [[weakness]]. Patients who develop symptoms of hyperglycemia during treatment with atypical antipsychotics should undergo fasting blood glucose testing. In some cases, hyperglycemia has resolved when the atypical antipsychotic was discontinued; however, some patients require continuation of anti-diabetic treatment despite discontinuation of the suspect drug. | |||
* | |||
::*In the long-term, open-label [[schizophrenia]] study with Aripiprazole lauroxil, 14% of patients with normal hemoglobin [[A1c]] (<5.7%) at baseline developed elevated levels (≥5.7%) post-baseline. | |||
* | |||
:*'''[[Dyslipidemia]]''' | |||
* | |||
[[ | ::*Undesirable alterations in [[lipids]] have been observed in patients treated with atypical [[antipsychotics]]. | ||
* | ::*In the long-term, open-label [[schizophrenia]] study with Aripiprazole lauroxil, shifts in baseline fasting total [[cholesterol]] from normal (<200 mg/dL) to high (≥240 mg/dL) were reported in 1% of patients; shifts in baseline fasting [[LDL]] cholesterol from normal (<100 mg/dL) to high (≥160 mg/dL) were reported in 1% of patients; and shifts in baseline fasting [[triglycerides]] from normal (<150 mg/dL) to high (≥200 mg/dL) were reported in 8% of patients. In the same study, shifts in baseline fasting total [[cholesterol]] from borderline (≥ 200 mg/dL and <240 mg/dL) to high (≥240 mg/dL) were reported in 15% of patients; shifts in baseline fasting [[LDL]] cholesterol from borderline (≥100 mg/dL and <160 mg/dL) to high (≥160 mg/dL) were reported in 8% of patients; and shifts in baseline fasting [[triglycerides]] from borderline (≥150 mg/dL and <200 mg/dL) to high (≥200 mg/dL) were reported in 35% of patients. In addition, the proportion of patients with shifts in fasting HDL [[cholesterol]] from normal (≥40 mg/dL) to low (<40 mg/dL) was reported in 15% of patients. | ||
:*'''Weight Gain''' | |||
::*Weight gain has been observed with atypical [[antipsychotic]] use. Clinical monitoring of weight is recommended. | |||
* | |||
::*The proportion of adult patients with weight gain ≥7% of body weight is presented in TABLE 6. | |||
* | :*'''Table 6: Proportion of Adult Patients with Shifts in Weight in the 12-Week, Placebo-Controlled, Fixed-Dose Schizophrenia Trial''' | ||
[[File:table7_ari.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
<SMALL>ARISTADA: Aripiprazole lauroxil's Brand name</SMALL> | |||
===== | ======Pathological Gambling and Other Compulsive Behaviors====== | ||
Post-marketing case reports suggest that patients can experience intense urges, particularly for gambling, and the inability to control these urges while taking aripiprazole. Other compulsive urges, reported less frequently include: sexual urges, shopping, eating or binge eating, and other impulsive or compulsive behaviors. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to ask patients or their caregivers specifically about the development of new or intense gambling urges, compulsive sexual urges, compulsive shopping, binge or compulsive eating, or other urges while being treated with aripiprazole. It should be noted that impulse-control symptoms can be associated with the underlying disorder. In some cases, although not all, urges were reported to have stopped when the dose was reduced or the medication was discontinued. Compulsive behaviors may result in harm for the patient and others if not recognized. Consider dose reduction or stopping the medication if a patient develops such urges. | |||
====== | ======[[Orthostatic Hypotension]]====== | ||
Aripiprazole may cause orthostatic hypotension, perhaps due to its [[α1-adrenergic receptor]] antagonism. Associated adverse reactions related to orthostatic hypotension can include [[dizziness]], [[lightheadedness]] and [[tachycardia]]. Generally, these risks are greatest at the beginning of treatment and during dose escalation. Patients at increased risk of these adverse reactions or at increased risk of developing complications from [[hypotension]] include those with [[dehydration]], [[hypovolemia]], treatment with [[antihypertensive]] medication, history of cardiovascular disease (e.g., [[heart failure]], [[myocardial infarction]], [[ischemia]], or [[conduction abnormalities]]), history of [[cerebrovascular disease]], as well as patients who are antipsychotic-naïve. In such patients, consider using a lower starting dose, and monitor orthostatic vital signs. | |||
[[ | Orthostatic hypotension was reported for one patient in the Aripiprazole lauroxil 882 mg group (0.5%) and no patients in the Aripiprazole lauroxil 441 mg and [[placebo]] groups in the 12-week [[schizophrenia]] efficacy study. In the long-term open-label schizophrenia study, orthostatic hypotension was reported for 1 (0.2%) patient treated with Aripiprazole lauroxil. Orthostatic hypotension was defined as a decrease in [[systolic blood pressure]] ≥20 mmHg accompanied by an increase in [[heart rate]] ≥25 bpm when comparing standing to [[supine]] values. | ||
======[[Leukopenia]], [[Neutropenia]], and [[Agranulocytosis]]====== | |||
In clinical trials and/or postmarketing experience, events of leukopenia and neutropenia have been reported temporally related to [[antipsychotic]] agents. Agranulocytosis has also been reported. | |||
[[ | Possible risk factors for leukopenia/neutropenia include pre-existing low [[white blood cell]] count (WBC)/absolute [[neutrophil]] count (ANC) and history of drug-induced leukopenia/neutropenia. In patients with a history of a clinically significant low WBC/ANC or drug-induced leukopenia/neutropenia, perform a [[complete blood count]] (CBC) frequently during the first few months of therapy. In such patients, consider discontinuation of Aripiprazole lauroxil at the first sign of a clinical significant decline in WBC in the absence of other causative factors. | ||
Monitor patients with clinically significant neutropenia for fever or other symptoms or signs of infection and treat promptly if such symptoms or signs occur. Discontinue Aripiprazole lauroxil in patients with severe neutropenia (absolute neutrophil count <1000/mm3) and follow their WBC until recovery. | |||
[[ | ======[[Seizures]]====== | ||
As with other [[antipsychotic]] drugs, use Aripiprazole lauroxil cautiously in patients with a history of seizures or with conditions that lower the seizure threshold. Conditions that lower the seizure threshold may be more prevalent in a population of 65 years or older. | |||
======Potential for Cognitive and Motor Impairment====== | |||
Aripiprazole lauroxil, like other [[antipsychotics]], has the potential to impair judgment, thinking or motor skills. Patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that therapy with Aripiprazole lauroxil does not affect them adversely. | |||
[[ | ======Body Temperature Regulation====== | ||
Disruption of the body's ability to reduce [[core body temperature]] has been attributed to [[antipsychotic]] agents. Appropriate care is advised when prescribing Aripiprazole lauroxil for patients who will be experiencing conditions which may contribute to an elevation in core body temperature, (e.g., exercising strenuously, exposure to extreme heat, receiving concomitant medication with [[anticholinergic]] activity, or being subject to [[dehydration]]). | |||
===== | ======[[Dysphagia]]====== | ||
[[Esophageal dysmotility]] and [[aspiration]] have been associated with [[antipsychotic]] drug use. Aripiprazole lauroxil and other antipsychotic drugs should be used cautiously in patients at risk for [[aspiration pneumonia]]. | |||
|clinicalTrials= | |||
The following are discussed in more details in other sections of the labeling: | |||
[[ | :*Increased Mortality in Elderly Patients with [[Dementia]]-related [[Psychosis]] | ||
:*Cerebrovascular Adverse Reactions, Including [[Stroke]] | |||
:*[[Neuroleptic Malignant Syndrome]] | |||
:*[[Tardive Dyskinesia]] | |||
:*Metabolic Changes | |||
:*Pathological Gambling and Other Compulsive Behaviors | |||
:*[[Orthostatic Hypotension]] | |||
:*[[Leukopenia]], [[Neutropenia]], and [[Agranulocytosis]] | |||
:*[[Seizures]] | |||
:*Potential for Cognitive and Motor Impairment | |||
:*Body Temperature Regulation | |||
:*[[Dysphagia]] | |||
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |||
======Aripiprazole lauroxil====== | |||
* | :*Patient Exposure: Aripiprazole lauroxil has been evaluated for safety in 880 adult patients in clinical trials in [[schizophrenia]]. | ||
:*Commonly Observed Adverse Reactions: The most common adverse reaction (incidence ≥5% and at least twice the rate of placebo in patients treated with Aripiprazole lauroxil) was [[akathisia]]. | |||
* | |||
:*Adverse Reactions Occurring at an Incidence of 2% or More in Aripiprazole lauroxil-Treated Patients: Adverse reactions associated with the use of Aripiprazole lauroxil (incidence of 2% or greater, rounded to the nearest percent and Aripiprazole lauroxil incidence greater than [[placebo]]) that occurred are shown in TABLE 7. | |||
* | |||
:*'''Table 7: Adverse Reaction in 2% or More of Aripiprazole lauroxil-Treated Patients and That Occurred at Greater Incidence than in the Placebo-Treated Patients in the 12-Week, Placebo-Controlled, Fixed-Dose Schizophrenia Trial''' | |||
* | [[File:table8_ari.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
:*Injection Site Reactions: Injection site reactions were reported by 4% of patients treated with 441 mg Aripiprazole lauroxil and 5% of patients treated with 882 mg Aripiprazole lauroxil compared to 2% of patients treated with [[placebo]]. Most of these were injection site pain (3%, 4% and 2% in the 441 mg Aripiprazole lauroxil, 882 mg Aripiprazole lauroxil and placebo groups, respectively) and most were associated with the first injection, and decreased with each subsequent injection to less than or equal to 1% for both doses of Aripiprazole lauroxil and placebo. Other injection site reactions ([[induration]], swelling and redness) occurred at less than 1%. | |||
* | |||
:*[[Extrapyramidal Symptoms]]: In the 12-week [[schizophrenia]] efficacy study, for Aripiprazole lauroxil-treated patients, the incidence of other EPS-related events, excluding [[akathisia]] and [[restlessness]], was 5% and 7% for patients on 441 mg and 882 mg, respectively, versus 4% for placebo-treated patient (TABLE 8). | |||
* | |||
:*'''Table 8: Incidence of EPS Compared to Placebo''' | |||
* | [[File:table9_ari.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
<SMALL>ARISTADA: Aripiprazole lauroxil's Brand name</SMALL> | |||
:*[[Dystonia]]: Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: [[spasm]] of the neck muscles, sometimes progressing to [[tightness]] of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation [[antipsychotic]] drugs. An elevated risk of acute dystonia is observed in males and younger age groups. | |||
* | |||
:*Other Adverse Reactions Observed in Clinical Studies: The following listing does not include reactions: 1) already listed in previous tables or elsewhere in labeling, 2) for which a drug cause was remote, 3) which were so general as to be uninformative, 4) which were not considered to have significant clinical implications, or 5) which occurred at a rate equal to or less than placebo. | |||
::*Cardiac – [[angina pectoris]], [[tachycardia]], [[palpitations]] | |||
:* | ::*Gastrointestinal disorders – [[constipation]], [[dry mouth]] | ||
: | ::*General disorders – [[asthenia]] | ||
:* | |||
::*Musculoskeletal – [[muscular weakness]] | |||
: | |||
:* | ::*Nervous system disorders – [[dizziness]] | ||
: | ::*Psychiatric disorders – [[anxiety]], [[suicide]] | ||
:* | |||
: | |||
:* | |||
:* | |||
======Adverse Reactions Reported in Clinical Trials with Oral Aripiprazole====== | |||
The following is a list of additional adverse reactions that have been reported in clinical trials with oral aripiprazole and not reported above for Aripiprazole lauroxil. | |||
[[ | :*Blood and Lymphatic System Disorders: [[thrombocytopenia]] | ||
* | :*Cardiac Disorders: [[bradycardia]], [[atrial flutter]], [[cardiorespiratory arrest]], [[atrioventricular block]], [[atrial fibrillation]], [[myocardial ischemia]], [[myocardial infarction]], [[cardiopulmonary failure]] | ||
[[ | :*Eye Disorders: [[photophobia]], [[diplopia]] | ||
:*Gastrointestinal Disorders: [[gastroesophageal reflux disease]] | |||
* | |||
[[ | :*General Disorders and Administration Site Conditions: [[peripheral edema]], [[chest pain]], face [[edema]] | ||
:*Hepatobiliary Disorders: [[hepatitis]], [[jaundice]] | |||
* | |||
[[ | :*Immune System Disorders: [[hypersensitivity]] | ||
:*Injury, Poisoning, and Procedural Complications: fall, [[heat stroke]] | |||
* | |||
:*Investigations: weight decreased, hepatic [[enzyme]] increased, blood glucose increased, blood [[lactate dehydrogenase]] increased, [[gamma glutamyl transferase]] increased, blood [[prolactin]] increased, blood [[urea]] increased, blood [[creatinine]] increased, blood [[bilirubin]] increased, [[electrocardiogram]] [[QT]] prolonged, [[glycosylated hemoglobin]] increased | |||
[[ | :*Metabolism and Nutrition Disorders: [[anorexia]], [[hypokalemia]], [[hyponatremia]], [[hypoglycemia]] | ||
:*Musculoskeletal and Connective Tissue Disorders: [[muscle tightness]], [[rhabdomyolysis]], mobility decreased | |||
* | |||
[[ | :*Nervous System Disorders: memory impairment, [[cogwheel rigidity]], [[hypokinesia]], [[myoclonus]], [[bradykinesia]], [[akinesia]], [[myoclonus]], coordination abnormal, [[speech disorder]], [[choreoathetosis]] | ||
:*Psychiatric Disorders: aggression, loss of [[libido]], [[delirium]], libido increased, [[anorgasmia]], [[tic]], homicidal ideation, [[catatonia]], [[sleep walking]] | |||
* | |||
[[ | :*Renal and Urinary Disorders: [[urinary retention]], [[nocturia]] | ||
:*Reproductive System and Breast Disorders: [[erectile dysfunction]], [[gynaecomastia]], menstruation irregular, [[amenorrhea]], [[breast pain]], [[priapism]] | |||
* | |||
:*Respiratory, Thoracic, and Mediastinal Disorders: [[nasal congestion]], [[dyspnea]] | |||
* | :*Skin and Subcutaneous Tissue Disorders: [[rash]], [[hyperhidrosis]], [[pruritus]], [[photosensitivity]] reaction, [[alopecia]], [[urticaria]] | ||
:*Vascular Disorders: [[hypotension]], [[hypertension]] | |||
|postmarketing=The following adverse reactions have been identified during post-approval use of oral aripiprazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or to establish a causal relationship to drug exposure: occurrences of allergic reaction ([[anaphylactic]] reaction, [[angioedema]], [[laryngospasm]], [[pruritus]]/[[urticaria]], or [[oropharyngeal]] [[spasm]]), pathological gambling, [[hiccups]] and blood glucose fluctuation. | |||
|drugInteractions= | |||
* | ======Drugs Having Clinically Important Interactions with Aripiprazole lauroxil====== | ||
:*'''Table 9: Clinically Important Drug Interactions with Aripiprazole lauroxil''' | |||
[[File:table10_ari.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
<SMALL>ARISTADA: Aripiprazole lauroxil's Brand name</SMALL> | |||
===== | ======Drugs Having No Clinically Important Interactions with Aripiprazole lauroxil====== | ||
Based on [[pharmacokinetic]] studies with oral aripiprazole, no dosage adjustment of Aripiprazole lauroxil is required when administered concomitantly with [[famotidine]], [[valproate]], [[lithium]]. | |||
In addition, no dosage adjustment is necessary for substrates of [[CYP2D6]] (e.g., [[dextromethorphan]], [[fluoxetine]], [[paroxetine]], or [[venlafaxine]]), [[CYP2C9]] (e.g., [[warfarin]]), [[CYP2C19]] (e.g., [[omeprazole]], [[warfarin]], [[escitalopram]]), or CYP3A4 (e.g., [[dextromethorphan]]) when co-administered with Aripiprazole lauroxil. Additionally, no dosage adjustment is necessary for [[valproate]], [[lithium]], [[lamotrigine]], or [[sertraline]] when co-administered with Aripiprazole lauroxil. | |||
== | |FDAPregCat= | ||
|useInPregnancyFDA=N | |||
:*'''Pregnancy Exposure Registry''' | |||
* | ::*There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Aripiprazole lauroxil during pregnancy. For more information, contact the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or visit http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/ . | ||
:*'''Risk Summary''' | |||
* | ::*Neonates exposed to [[antipsychotic]] drugs during the third trimester of pregnancy are at risk for [[extrapyramidal]] and/or withdrawal symptoms following delivery. Limited published data on aripiprazole use in pregnant women are not sufficient to inform any drug-associated risks for birth defects or [[miscarriage]]. No [[teratogenicity]] was observed in animal reproductive studies with [[intramuscular]] administration of aripiprazole lauroxil to rats and rabbits during [[organogenesis]] at doses up to 6 and 18 times, respectively, the maximum recommended human dose (MRHD) of 882 mg based on [[body surface area]] (mg/m2). However, aripiprazole caused developmental [[toxicity]] and possible [[teratogenic]] effects in rats and rabbits. The background risk of major birth defects and [[miscarriage]] for the indicated population are unknown. In the U.S. general population, the estimated background risk of major birth defects and [[miscarriage]] in clinically recognized pregnancies is 2-4% and 15-20%, respectively. Advise pregnant women of the potential risk to a fetus. | ||
:*'''Clinical Considerations''' | |||
::*Fetal/Neonatal Adverse Reactions | |||
* | |||
:::*Extrapyramidal and/or withdrawal symptoms, including [[agitation]], [[hypertonia]], [[hypotonia]], [[tremor]], [[somnolence]], [[respiratory distress]] and [[feeding disorder]] have been reported in neonates who were exposed to [[antipsychotic]] drugs during the third trimester of pregnancy. These symptoms have varied in severity. Monitor neonates for [[extrapyramidal]] and/or withdrawal symptoms and manage symptoms appropriately. Some neonates recover within hours or days without specific treatment; others required prolonged hospitalization. | |||
* | |||
:*'''Data''' | |||
* | |||
::*Animal Data for Aripiprazole Lauroxil | |||
* | |||
:::*Aripiprazole lauroxil did not cause adverse developmental or maternal effects in rats or rabbits when administered intramuscularly during the period of [[organogenesis]] at doses of 18, 49, or 144 mg/animal in pregnant rats which are approximately 0.7 to 6 times the maximum recommended human dose (MRHD) of 882 mg on mg/m2 basis, and at doses of 241, 723, and 2893 mg/animal in pregnant rabbits which are approximately 1 to 18 times the MRHD on mg/m2 basis. However, aripiprazole caused developmental toxicity and possible [[teratogenic]] effects in rats and rabbits. | |||
* Aripiprazole at doses of | |||
::*Animal Data for Aripiprazole | |||
* Aripiprazole | |||
:::*Pregnant rats were treated with oral doses of 3, 10, and 30 mg/kg/day which are approximately 1 to 10 times the oral maximum recommended human dose [MRHD] of 30 mg/day on mg/m2 basis of aripiprazole during the period of [[organogenesis]]. Treatment at the highest dose caused a slight prolongation of gestation and delay in fetal development, as evidenced by decreased fetal weight, and undescended testes. Delayed skeletal [[ossification]] was observed at 3 and 10 times the oral MRHD on mg/m2 basis. | |||
* | |||
:::*At 3 and 10 times the oral MRHD on mg/m2 basis, delivered offspring had decreased body weights. Increased incidences of [[hepatodiaphragmatic nodules]] and [[diaphragmatic hernia]] were observed in offspring from the highest dose group (the other dose groups were not examined for these findings). A low incidence of diaphragmatic hernia was also seen in the fetuses exposed to the highest dose. Postnatally, delayed vaginal opening was seen at 3 and 10 times the oral MRHD on mg/m2 basis and impaired reproductive performance (decreased fertility rate, [[corpora lutea]], implants, [[live fetuses]], and increased [[post-implantation loss]], likely mediated through effects on female offspring) along with some maternal toxicity were seen at the highest dose; however, there was no evidence to suggest that these developmental effects were secondary to maternal toxicity. | |||
* | |||
:::*In pregnant rabbits treated with oral doses of 10, 30, and 100 mg/kg/day which are 2 to 11 times human exposure at the oral MRHD based on [[AUC]] and 6 to 65 times the oral MRHD on mg/m2 basis of aripiprazole during the period of [[organogenesis]] decreased maternal food consumption and increased [[abortions]] were seen at the highest dose as well as increased fetal mortality. Decreased fetal weight and increased incidence of fused sternebrae were observed at 3 and 11 times the oral MRHD based on AUC. | |||
* | |||
:::*In rats treated with oral doses of 3, 10, and 30 mg/kg/day which are 1 to 10 times the oral MRHD on mg/m2 basis of aripiprazole perinatally and postnatally (from day 17 of gestation through day 21 postpartum), slight maternal toxicity and slightly prolonged gestation were seen at the highest dose. An increase in stillbirths and decreases in pup weight (persisting into adulthood) and survival were also seen at this dose. | |||
* | |||
= | |useInNursing=Aripiprazole is present in human breast milk; however, there are insufficient data to assess the amount in human milk, the effects on the breastfed infant, or the effects on milk production. The development and health benefits of breastfeeding should be considered along with the mother's clinical need for Aripiprazole lauroxil and any potential adverse effects on the breastfed infant from Aripiprazole lauroxil or from the underlying maternal condition. | ||
|useInPed=Safety and effectiveness of Aripiprazole lauroxil in patients <18 years of age have not been evaluated. | |||
|useInGeri=Safety and effectiveness of Aripiprazole lauroxil in patients >65 years of age have not been evaluated. | |||
|useInRenalImpair=No dosage adjustment for Aripiprazole lauroxil is required based on a patient's [[renal function]] (mild to severe renal impairment, [[glomerular filtration rate]] between 15 and 90 mL/minute). | |||
|useInHepaticImpair=No dosage adjustment for Aripiprazole lauroxil is required based on a patient's [[hepatic function]] (mild to severe hepatic impairment, [[Child-Pugh]] score between 5 and 15). | |||
| | |othersTitle=CYP2D6 Poor Metabolizers | ||
|useInOthers=Dosage adjustment is recommended in known [[CYP2D6]] poor metabolizers due to high aripiprazole concentrations. Approximately 8% of Caucasians and 3-8% of Black/African Americans cannot metabolize CYP2D6 substrates and are classified as poor metabolizers (PM). | |||
== | |othersTitle=Other Specific Populations | ||
|useInOthers=No dosage adjustment for Aripiprazole lauroxil is required on the basis of a patient's sex, race, or smoking status. | |||
|administration= | |administration= | ||
* | :*'''Instructions for Use''' | ||
* | ::*The kit contains a syringe containing Aripiprazole lauroxil sterile aqueous suspension and 2 or 3 safety needles depending on dose (a 2-inch 20 gauge needle with yellow needle hub, a 1 ½-inch 20 gauge needle with yellow needle hub, and a 1-inch 21 gauge needle with green needle hub (441 mg kit only)) for [[intramuscular]] injection. All materials should be stored at room temperature. | ||
[[File:ari1.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
<SMALL>ARISTADA: Aripiprazole lauroxil's Brand name</SMALL> | |||
::'''1.''' '''TAP''' and '''SHAKE''' the syringe. | |||
[[File:ari2.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
:::'''1a.''' '''Tap''' the syringe at least 10 times to dislodge any material which may have settled. | |||
:::'''1b.''' '''Shake''' the syringe vigorously for a minimum of 30 seconds to ensure a uniform suspension. If the syringe is not used within 15 minutes, shake again for 30 seconds. | |||
::'''2.''' '''SELECT''' the injection needle. | |||
:::'''2a.''' '''Select''' injection site. | |||
:::'''2b.''' '''Select''' needle length based on injection site. For patients with a larger amount of subcutaneous tissue overlaying the injection site muscle, use the longer of the needles provided. | |||
| | :*'''Table 5: Injection Site and Associated Needle Length''' | ||
[[File:table5_ari.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
::'''3.''' '''ATTACH''' the injection needle. | |||
:::'''Attach''' the appropriate needle securely with a clockwise twisting motion. Do NOT overtighten. Overtightening could lead to needle hub cracking. | |||
[[File:ari3.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
::'''4.''' [[PRIME]] the syringe to remove air. | |||
:::'''4a.''' '''Bring''' the syringe into upright position and tap the syringe to bring air to the top. | |||
[[File:ari4.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
:::'''4b.''' '''Remove''' air by depressing the plunger rod. A few drops of suspension will be released. | |||
[[File:ari5.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
::'''5.''' '''ADMINISTER''' the entire content intramuscularly. Do not inject by any other route. Inject in a rapid and continuous manner (less than 10 seconds). | |||
[[File:ari6.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
::'''6.''' '''DISPOSE''' of the needle. Cover the needle by pressing the safety device. Dispose of used and unused items in a proper waste container. | |||
[[File:ari7.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|overdose= | |||
:*'''Human Experience''' | |||
::*The largest known case of acute ingestion with a known outcome involved 1260 mg of oral aripiprazole (42 times the maximum recommended daily dose) in a patient who fully recovered. | |||
::*Common adverse reactions (reported in at least 5% of all overdose cases) reported with oral aripiprazole overdosage (alone or in combination with other substances) include [[vomiting]], [[somnolence]], and [[tremor]]. Other clinically important signs and symptoms observed in one or more patients with aripiprazole overdoses (alone or with other substances) include [[acidosis]], [[aggression]], [[aspartate aminotransferase]] increased, [[atrial fibrillation]], [[bradycardia]], [[coma]], [[confusional state]], [[convulsion]], blood [[creatine phosphokinase]] increased, depressed level of consciousness, [[hypertension]], [[hypokalemia]], [[hypotension]], [[lethargy]], loss of consciousness, [[QRS]] complex prolonged, [[QT]] prolonged, [[pneumonia aspiration]], [[respiratory arrest]], [[status epilepticus]], and [[tachycardia]]. | |||
:*'''Management of Overdosage''' | |||
::*In case of overdosage, call the Poison control center immediately at 1-800-222-1222. | |||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| Verifiedfields = | |||
| Watchedfields = | |||
| verifiedrevid = | |||
| IUPAC_name = [7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-2-oxo-3,4-dihydroquinolin-1-yl]methyl dodecanoate | |||
| image = ari15.png | |||
| width = | |||
<!--Clinical data--> | <!--Clinical data--> | ||
| tradename = | | tradename = Aristada | ||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| pregnancy_category = | |||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | |||
| pregnancy_AU = B3 | | legal_CA = | ||
| pregnancy_US = C | | legal_UK = | ||
| legal_AU = S4 | | legal_US = | ||
| legal_CA = | | legal_status = | ||
| legal_US = | | routes_of_administration = [[Intramuscular injection|Intramuscular]] | ||
| | |||
| routes_of_administration = | |||
<!--Pharmacokinetic data--> | <!--Pharmacokinetic data--> | ||
| bioavailability = | | bioavailability = | ||
| protein_bound = | | protein_bound = | ||
| metabolism = | | metabolism = | ||
| elimination_half-life = | | elimination_half-life = | ||
| excretion = | | excretion = | ||
<!--Identifiers--> | <!--Identifiers--> | ||
| CAS_number_Ref = | |||
| CAS_number_Ref = | | CAS_number = 1259305-29-7 | ||
| CAS_number = | | CAS_supplemental = | ||
| ATC_prefix = | | ATC_prefix = | ||
| ATC_suffix = | | ATC_suffix = | ||
| PubChem = | | PubChem = 49831411 | ||
| DrugBank_Ref = | |||
| DrugBank_Ref = | | DrugBank = | ||
| DrugBank = | | ChemSpiderID_Ref = | ||
| ChemSpiderID_Ref = | | ChemSpiderID = 28651973 | ||
| ChemSpiderID = | |||
<!--Chemical data--> | <!--Chemical data--> | ||
| C= | | C=36 | H=51 | Cl=2 | N=3 | O=4 | ||
| molecular_weight = | | molecular_weight = 660.71384 g/mol | ||
| smiles = | | smiles = CCCCCCCCCCCC(=O)OCN1C(=O)CCC2=C1C=C(C=C2)OCCCCN3CCN(CC3)C4=C(C(=CC=C4)Cl)Cl | ||
| | | StdInChI = 1S/C36H51Cl2N3O4/c1-2-3-4-5-6-7-8-9-10-16-35(43)45-28-41-33-27-30(19-17-29(33)18-20-34(41)42)44-26-12-11-21-39-22-24-40(25-23-39)32-15-13-14-31(37)36(32)38/h13-15,17,19,27H,2-12,16,18,20-26,28H2,1H3 | ||
| StdInChIKey = DDINXHAORAAYAD-UHFFFAOYSA-N | |||
| synonyms = ALKS-9070, ALKS-9072, RDC-3317 | |||
| | |||
| | |||
}} | }} | ||
:*In | |mechAction=Aripiprazole lauroxil is a [[prodrug]] of aripiprazole. Following [[intramuscular]] injection, Aripiprazole lauroxil is likely converted by [[enzyme]]-mediated [[hydrolysis]] to N-hydroxymethyl aripiprazole, which is then hydrolyzed to aripiprazole. The [[mechanism of action]] of aripiprazole in the body is unknown. However, efficacy could be mediated through a combination of partial agonist activity [[D2]] and [[5-HT1A]] receptors and antagonist activity at [[5-HT2A]] receptors. Actions at receptors other than D2, 5-HT1A, and 5-HT2A could explain some of the adverse reactions of aripiprazole (e.g., the [[orthostatic hypotension]] observed with aripiprazole may be explained by its antagonist activity at adrenergic [[alpha1 receptor]]s). | ||
|structure= | |||
Aripiprazole lauroxil is an atypical [[antipsychotic]]. | |||
The chemical name of Aripiprazole lauroxil is 7-{4-[4-(2,3-dichlorophenyl)-piperazin-1-yl]butoxy}-2-oxo-3,4-dihydro-2H-quinolin-1-yl)methyl dodecanoate. The empirical formula is C36H51Cl2N3O4 and its molecular weight is 660.7 g/mol. The chemical structure is: | |||
[[File:ari8.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Aripiprazole lauroxil is available as a white to off-white sterile aqueous extended-release suspension for [[intramuscular]] injection in the following strengths of aripiprazole lauroxil (and deliverable volumes from a single-use pre-filled syringe): 441 mg (1.6 mL), 662 mg (2.4 mL) and 882 mg (3.2 mL). The inactive ingredients include sorbitan monolaurate (3.8 mg/mL), polysorbate 20 (1.5 mg/mL), sodium chloride (6.1 mg/mL), sodium phosphate dibasic anhydrous, sodium phosphate monobasic and water for injection. | |||
|PD=Aripiprazole exhibits high affinity for [[dopamine]] D2 and D3, [[serotonin]] [[5-HT1A]] and [[5-HT2A]] receptors (Ki values 0.34 nM, 0.8 nM, 1.7 nM, and 3.4 nM, respectively), moderate affinity for dopamine D4, serotonin [[5-HT2C]] and [[5-HT7]], [[alpha1-adrenergic]] and [[histamine]] H1 receptors (Ki values 44 nM, 15 nM, 39 nM, 57 nM, and 61 nM, respectively), and moderate affinity for the [[serotonin]] reuptake site (Ki =98 nM). Aripiprazole has no appreciable affinity for [[cholinergic muscarinic receptor]]s (IC50>1000 nM). Aripiprazole functions as a partial agonist at the dopamine D2 and the serotonin 5-HT1A receptors, and as an antagonist at serotonin 5-HT2A receptor. | |||
|PK= | |||
Aripiprazole lauroxil is a [[prodrug]] of aripiprazole and its activity in the body is primarily due to aripiprazole, and to a lesser extent dehydro-aripiprazole (major [[metabolite]] of aripiprazole), which has been shown to have affinities for D2 receptors similar to aripiprazole and represents 30-40% of the aripiprazole exposure in plasma. | |||
:*'''[[Absorption]] and [[Distribution]]''' | |||
::*After single [[intramuscular]] injection the appearance of aripiprazole in the systemic circulation starts from 5 to 6 days and continues to be released for an additional 36 days. Aripiprazole concentrations increase with consecutive doses of Aripiprazole lauroxil and reach steady-state following the fourth monthly injection. The concentration-time course of dehydro-aripiprazole followed that of aripiprazole. | |||
::*With the addition of oral aripiprazole supplementation for 21 days at the time of the first Aripiprazole lauroxil dose, aripiprazole concentrations reach therapeutic levels within 4 days. | |||
::*Based on population [[pharmacokinetic]] analysis, the apparent [[volume of distribution]] of aripiprazole following [[intramuscular]] injection of Aripiprazole lauroxil was 268 L, indicating extensive [[extravascular]] distribution following absorption. At therapeutic concentrations, aripiprazole and its major [[metabolite]] are greater than 99% bound to serum proteins, primarily to [[albumin]]. In healthy human volunteers administered 0.5 mg/day to 30 mg/day oral aripiprazole for 14 days, there was dose-dependent D2 receptor occupancy indicating brain penetration of aripiprazole in humans. | |||
::*Aripiprazole exposure was similar for [[deltoid]] and [[gluteal]] intramuscular injections of 441 mg Aripiprazole lauroxil, thus are interchangeable. | |||
::*Administration of 882 mg every 6 weeks results in plasma aripiprazole concentrations that are within the established therapeutic range for 441 to 882 mg monthly. | |||
:*'''[[Metabolism]] and Elimination''' | |||
::*The [[biotransformation]] of Aripiprazole lauroxil likely involves [[enzyme]]-mediated [[hydrolysis]] to form N-hydroxymethyl-aripiprazole, which subsequently undergoes water mediated hydrolysis to aripiprazole. Elimination of aripiprazole is mainly through hepatic metabolism involving [[CYP3A4]] and [[CYP2D6]]. Dosage adjustments are recommended in CYP2D6 poor metabolizers due to high aripiprazole concentrations. | |||
::*The mean aripiprazole terminal elimination [[half-life]] ranged from 29.2 days to 34.9 days after every 4-week injection of Aripiprazole lauroxil 441, 662 and 882 mg. The significantly longer aripiprazole apparent half-life compared to oral aripiprazole (mean 75 hours) is attributed to the dissolution and formation rate-limited elimination of aripiprazole following Aripiprazole lauroxil administration. | |||
:*'''Drug Interaction Studies''' | |||
::*No specific drug interaction studies have been performed with Aripiprazole lauroxil. The drug interaction data provided below is obtained from studies with oral aripiprazole. | |||
::*Effects of other drugs on the exposures of aripiprazole and dehydro-aripiprazole are summarized in FIGURE 1 and FIGURE 2, respectively. Based on simulation, a 4.5-fold increase in mean [[Cmax]] and [[AUC]] values at steady-state is expected when extensive metabolizers of [[CYP2D6]] are administered with both strong [[CYP2D6]] and [[CYP3A4]] inhibitors. After oral administration, a 3-fold increase in mean Cmax and AUC values at steady-state is expected in poor metabolizers of CYP2D6 administered with strong CYP3A4 inhibitors. | |||
:*'''Figure 1: The Effects of Other Drugs on Aripiprazole Pharmacokinetics''' | |||
[[File:figure1_ari.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
:*'''Figure 2: The Effects of Other Drugs on Dehydro-aripiprazole Pharmacokinetics''' | |||
[[File:figure2_ari.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
The effects of aripiprazole on the exposures of other drugs are summarized in FIGURE 3. | |||
:*'''Figure 3: The Effects of Oral Aripiprazole on Pharmacokinetics of Other Drugs''' | |||
[[File:figure3_ari.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
:*'''Specific Population Studies''' | |||
::*A population [[pharmacokinetic]] analysis showed no effect of sex, race or smoking on Aripiprazole lauroxil pharmacokinetics. | |||
::*Exposures of aripiprazole and dehydro-aripiprazole using oral aripiprazole in specific populations are summarized in FIGURE 4 and FIGURE 5, respectively. | |||
:*'''Figure 4: Effects of Intrinsic Factors on Aripiprazole Pharmacokinetics''' | |||
[[File:figure4_ari.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
:*'''Figure 5: Effects of Intrinsic Factors on Dehydro-aripiprazole Pharmacokinetics''' | |||
[[File:figure5_ari.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|nonClinToxic=======[[Carcinogenesis]], [[Mutagenesis]], Impairment of [[Fertility]]====== | |||
:*'''Carcinogenesis''' | |||
::*Lifetime carcinogenicity studies have not been conducted with aripiprazole lauroxil. | |||
::*Lifetime carcinogenicity studies with oral aripiprazole have been conducted in ICR mice and in Sprague-Dawley (SD) and F344 rats. Aripiprazole was administered for 2 years in the diet at doses of 1, 3, 10, and 30 mg/kg/day to ICR mice and 1, 3, and 10 mg/kg/day to F344 rats (0.2 to 5 times and 0.3 to 3 times the oral maximum recommended human dose [MRHD] of 30 mg/day based on mg/m2, respectively). In addition, SD rats were dosed orally for 2 years at 10, 20, 40, and 60 mg/kg/day (3 to 19 times the oral MRHD based on mg/m2). Aripiprazole did not induce tumors in male mice or rats. In female mice, the incidences of [[pituitary gland]] [[adenomas]] and [[mammary gland]] [[adenocarcinomas]] and [[adenoacanthomas]] were increased at dietary doses which are 0.1 to 0.9 times human exposure at the oral MRHD based on [[AUC]] and 0.5 to 5 times the oral MRHD on mg/m2 basis. In female rats, the incidence of mammary gland [[fibroadenomas]] was increased at a dietary dose which is 0.1 times human exposure at the oral MRHD based on AUC and 3 times the oral MRHD on mg/m2 basis; and the incidences of [[adrenocortical carcinoma]]s and combined adrenocortical adenomas/carcinomas were increased at an oral dose which is 14 times human exposure at oral MRHD based on AUC and 19 times the oral MRHD on mg/m2 basis. | |||
::*Proliferative changes in the pituitary and mammary gland of rodents have been observed following chronic administration of other [[antipsychotic]] agents and are considered [[prolactin]]-mediated. The relevance for human risk of the findings of prolactin-mediated [[endocrine]] [[tumors]] in rodents is unknown. | |||
:*'''Mutagenesis''' | |||
::*Aripiprazole lauroxil was not mutagenic in the [[in vitro]] bacterial reverse mutation assay or [[clastogenic]] in the [[in vitro]] [[chromosome]] aberration assay in human [[peripheral blood]] [[lymphocytes]]. | |||
::*Aripiprazole and its [[metabolite]] (2,3-DCPP) were [[clastogenic]] in the [[in vitro]] [[chromosome]] aberration assay in Chinese hamster lung (CHL) cells both in the presence and absence of metabolic activation. The metabolite, 2,3-DCPP, produced increases in numerical aberrations in the in vitro assay in CHL cells in the absence of metabolic activation. A positive response was obtained in the oral [[in vivo]] micronucleus assay in mice; however, the response was due to a mechanism not considered relevant to humans. | |||
:*'''Impairment of Fertility''' | |||
::*Animal Data for Aripiprazole Lauroxil | |||
:::*In a rat fertility study, aripiprazole lauroxil was administered intramuscularly. Males were treated with doses of 18, 49, or 144 mg /animal, which are approximately 0.5 to 4 times the maximum recommended human dose [MRHD] of 882 mg on mg/m2 basis, on Days 1, 21, and 42 prior to and through mating; females were treated at these doses, which are approximately 0.7 to 6 times the MRHD on mg/m2 basis, once 14 days prior to mating. | |||
:::*In females, persistent diestrus was observed at all doses and the mean number of cycles was significantly decreased at the highest dose together with an increase in the copulatory interval (delay in mating). Additional changes at the high dose included slight increases in [[corpora lutea]] and pre-implantation loss, decline in mating, fertility, and fecundity indices in females and lower mating and fertility indices in males. | |||
::*Animal Data for Aripiprazole | |||
:::*Female rats were treated with oral aripiprazole doses of 2, 6, and 20 mg/kg/day, which are 0.6 to 6 times the oral maximum recommended human dose [MRHD] of 30 mg/day on mg/m2 basis, from 2 weeks prior to mating through day 7 of gestation. Estrous cycle irregularities and increased [[corpora lutea]] were seen at all doses, but no impairment of fertility was observed. Increased pre-implantation loss was found at 2 and 6 times the oral MRHD on mg/m2 basis and decreased fetal weight was noted at the highest dose which is 6 times the oral MRHD on mg/m2 basis. | |||
:::*Male rats were treated with oral aripiprazole doses of 20, 40, and 60 mg/kg/day, which are 6 to 19 times the oral MRHD on mg/m2 basis, from 9 weeks prior to and through mating. Disturbances in [[spermatogenesis]] at the highest dose and [[prostate]] atrophy at the mid and high doses were noted which are 13 and 19 times the oral MRHD on mg/m2 basis, but no impairment of fertility was observed. | |||
======Animal Toxicology and/or Pharmacology====== | |||
Intramuscular administration of aripiprazole lauroxil to rats and dogs was associated with injection site tissue reactions at all doses in rats treated up to 6 months at doses of 15, 29, and 103 mg/animal (which are approximately 0.3 to 2 times and 0.5 to 4 times the maximum recommended human dose [MRHD] of 882 mg on mg/m2 basis in males and females, respectively) and in dogs treated up to 9 months at doses of 147, 662, and 2058 mg/animal (which are approximately 0.5 to 8 times and 0.7 to 10 times the MRHD in males and females, respectively on mg/m2 basis). These injection site tissue reactions consisted of localized granulomatous inflammation and granuloma formation. Transiently impaired limb function and swelling occurred in dogs. The [[granulomas]] did not completely resolve 2 months following the last injection in the 6 month rat study and 4 months following the last injection in the 9 month dog study (the low dose groups were not examined for reversibility in these studies). | |||
Orally administered aripiprazole produced retinal degeneration in albino rats in a 26-week chronic toxicity study at a dose of 60 mg/kg, which is 19 times the oral MRHD of 30 mg/day on mg/m2 basis, and in a 2-year carcinogenicity study at doses of 40 mg/kg and 60 mg/kg, which are 13 and 19 times the oral MRHD on mg/m2 basis and 7 to 14 times human exposure at the oral MRHD based on [[AUC]]. Evaluation of the retinas of albino mice and of monkeys did not reveal evidence of [[retinal degeneration]]. Additional studies to further evaluate the mechanism have not been performed. The relevance of this finding to human risk is unknown. | |||
|clinicalStudies= | |||
The efficacy of Aripiprazole lauroxil in the treatment of patients with [[schizophrenia]] was established, in part, on the basis of efficacy data from trials with the oral formulation of aripiprazole. In addition, the efficacy of Aripiprazole lauroxil was established in a 12-week, randomized, double-blind, [[placebo]] controlled, fixed-dose study in adult patients with [[schizophrenia]] meeting DSM IV TR criteria [Study 1, n = 622; 207 (Aripiprazole lauroxil 441 mg), 208 (Aripiprazole lauroxil 882 mg), and 207 (placebo)]. After establishing tolerability to oral aripiprazole, patients received oral aripiprazole or placebo daily for the first 3 weeks. The [[intramuscular]] (IM) injections were administered on Days 1, 29 and 57. | |||
Efficacy was assessed using Positive and Negative Syndrome Scale (PANSS) and Clinical Global Impression Improvement Scale (CGI-I): | |||
:*The PANSS is a 30-item scale that measures positive symptoms of schizophrenia (7 items), negative symptoms of [[schizophrenia]] (7 items), and general [[psychopathology]] (16 items), each rated on a scale of 1 (absent) to 7 (extreme). Total PANSS scores range from 30 to 210. | |||
:*The CGI-I rates improvement in mental illness on a scale of 1 (very much improved) to 7 (very much worse) based on the change from baseline in clinical condition. | |||
Eligible patients were 18 to 70 years of age with PANSS total score of 70 to 120 and a score of ≥4 for at least 2 of the selected Positive Scale items. Patients were also required to have a CGI-S score of ≥4. | |||
The primary efficacy variable was the change from baseline to endpoint (Day 85) in PANSS total score. Statistically significant separation from placebo on PANSS total score change was observed in each Aripiprazole lauroxil dose group (TABLE 10). | |||
:*'''Table 10: Primary Efficacy Results''' | |||
[[File:table11_ari.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
<SMALL>ARISTADA: Aripiprazole lauroxil's Brand name</SMALL> | |||
The visit-wise mean change from baseline on PANSS total score change for each treatment group is shown in FIGURE 6. | |||
:*'''Figure 6: Change from Baseline in PANSS Total Score''' | |||
[[File:figure6_ari.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
<SMALL>ARISTADA: Aripiprazole lauroxil's Brand name</SMALL> | |||
|howSupplied= | |||
Aripiprazole lauroxil is a white to off-white aqueous extended-release suspension provided in a single-use pre-filled syringe for intramuscular injection in the deltoid or gluteal muscle at the 441 mg dose strength and in the gluteal muscle at dose strengths of 662 mg and 882 mg. | |||
Aripiprazole lauroxil extended-release injectable suspension is available in strengths of 441 mg in 1.6 mL, 662 mg in 2.4 mL, and 882 mg in 3.2 mL. The kit contains a 5-mL pre-filled syringe containing Aripiprazole lauroxil sterile aqueous suspension and safety needles. | |||
:*The 441 mg strength kit (NDC 65757-401-03; light blue label) contains three safety needles; a 1-inch (25 mm) 21 gauge, a 1½-inch (38 mm) 20 gauge, and a 2-inch (50 mm) 20 gauge needle. | |||
:*The 662 mg strength kit (NDC 65757-402-03; green label) contains two safety needles; a 1½-inch (38 mm) 20 gauge and a 2-inch (50 mm) 20 gauge needle. | |||
:*The 882 mg strength kit (NDC 65757-403-03; burgundy label) contains two safety needles; a 1½-inch (38 mm) 20 gauge and a 2-inch (50 mm) 20 gauge needle. | |||
Aripiprazole lauroxil is available as described in TABLE 11. | |||
:*'''Table 11: Presentations of Aripiprazole lauroxil''' | |||
[[File:table6_ari.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|storage=Store at room temperature 20°C to 25°C (68°F to 77°F) with excursions permitted between 15°C and 30°C (between 59°F and 86°F). | |||
|packLabel= | |||
[[File:ari9.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[File:ari10.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[File:ari11.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[File:ari12.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[File:ari13.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[File:ari14.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|fdaPatientInfo= | |||
Advise patients to read FDA-approved patient labeling (MEDICATION GUIDE). | |||
:*'''Pathological Gambling and Other Compulsive Behaviors''' | |||
::*Advise patients and their caregivers of the possibility that they may experience compulsive urges to shop, intense urges to gamble, compulsive sexual urges, binge eating and/or other compulsive urges and the inability to control these urges. In some cases, but not all, the urges were reported to have stopped when the dose was reduced or stopped. | |||
:*'''[[Neuroleptic Malignant Syndrome]]''' | |||
::*Counsel patients about a potentially fatal adverse reaction referred to as NMS that has been reported in association with administration of [[antipsychotic]] drugs. Advise patients to contact a healthcare provider or report to the emergency room if they experience signs or symptoms of NMS. | |||
:*'''[[Tardive Dyskinesia]]''' | |||
::*Advise patients that abnormal involuntary movements have been associated with administration of [[antipsychotic]] drugs. Counsel patients to notify their healthcare provider if they notice any movements which they cannot control in their face, tongue, or other body part. | |||
:*'''Metabolic Changes ([[Hyperglycemia]] and [[Diabetes Mellitus]], [[Dyslipidemia]], and Weight Gain)''' | |||
:* | ::*Educate patients about the risk of metabolic changes, how to recognize symptoms of hyperglycemia and diabetes mellitus, and the need for specific monitoring, including blood glucose, lipids, and weight. | ||
:* | :*'''[[Orthostatic Hypotension]]''' | ||
:* | ::*Educate patients about the risk of orthostatic hypotension (symptoms include feeling [[dizzy]] or [[lightheaded]] upon standing), particularly at the time of initiating treatment, re-initiating treatment, or increasing the dose. | ||
:*'''[[Leukopenia]]/ [[Neutropenia]]''' | |||
:* | |||
:* | ::*Advise patients with a pre-existing low [[WBC]] count or a history of drug-induced leucopenia/neutropenia that they should have their [[CBC]] monitored while receiving Aripiprazole lauroxil. | ||
:*'''Interference with Cognitive and Motor Performance''' | |||
* | ::*Because Aripiprazole lauroxil may have the potential to impair judgment, thinking or motor skills, instruct patients to be cautious about operating hazardous machinery, including automobiles, until they are reasonably certain that Aripiprazole lauroxil therapy does not affect them adversely. | ||
:*'''Heat Exposure and [[Dehydration]]''' | |||
:* | ::*Advise patients regarding appropriate care in avoiding overheating and dehydration. | ||
:*'''Concomitant Medication''' | |||
: | |||
::*Advise patients to inform their physicians if they are taking, or plan to take, any prescription or over-the-counter drugs, since there is a potential for interactions. | |||
:*'''Pregnancy''' | |||
* | |||
::*Advise patients that Aripiprazole lauroxil may cause extrapyramidal and/or withdrawal symptoms in a neonate and to notify their healthcare provider with a known or suspected pregnancy. Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Aripiprazole lauroxil during pregnancy. | |||
[[File:ari16.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
* | |||
* | |brandNames=ARISTADA® | ||
* | |||
* | |||
| | |||
| | |||
| | |||
| | |||
| | |||
}} | }} | ||
Latest revision as of 19:54, 16 February 2017
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Martin Nino [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
See full prescribing information for complete Boxed Warning.
|
Overview
Aripiprazole (intramuscular) is an atypical antipsychotic that is FDA approved for the treatment of patients with schizophrenia. There is a Black Box Warning for this drug as shown here. Common adverse reactions include akathisia (≥5%).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Aripiprazole lauroxil is indicated for treatment of schizophrenia.
Dosage
- Treatment of Schizophrenia
- Aripiprazole lauroxil is only to be administered as an intramuscular injection by a healthcare professional. For patients who have never taken aripiprazole, establish tolerability with oral aripiprazole prior to initiating treatment with Aripiprazole lauroxil. Due to the half-life of oral aripiprazole, it may take up to 2 weeks to fully assess tolerability. Refer to the prescribing information of oral aripiprazole for the recommended dosage and administration of the oral formulation.
- Depending on individual patient's needs, treatment with Aripiprazole lauroxil can be initiated at a dose of 441 mg, 662 mg or 882 mg administered monthly, which corresponds to 300 mg, 450 mg and 600 mg of aripiprazole, respectively. Treatment may also be initiated with the 882 mg dose every 6 weeks.
- Administer Aripiprazole lauroxil either in the deltoid muscle (441 mg dose only) or gluteal muscle (441 mg, 662 mg or 882 mg).
- Table 1: Aripiprazole lauroxil Dosing Frequency and Site of Injection
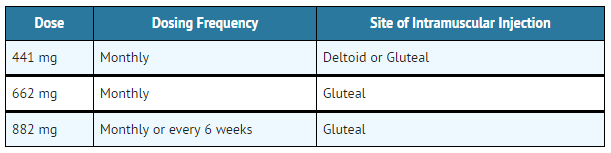
- Use the following Aripiprazole lauroxil doses for patients who are stabilized on oral aripiprazole, as shown in TABLE 2.
- Table 2: Aripiprazole lauroxil Doses Based on Oral Aripiprazole Total Daily Dose
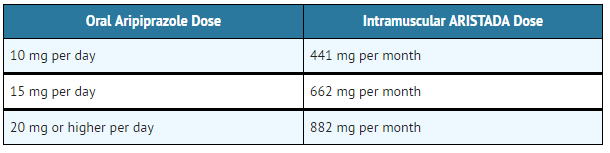
- In conjunction with the first Aripiprazole lauroxil injection, administer treatment with oral aripiprazole for 21 consecutive days.
- Dose may be adjusted as needed. When making dose and dosing interval adjustments, the pharmacokinetics and prolonged-release characteristics of Aripiprazole lauroxil should be considered.
- Missed Doses
- When a dose is missed, administer the next injection of Aripiprazole lauroxil as soon as possible. If the time elapsed since the last Aripiprazole lauroxil injection exceeds the length of time noted in TABLE 3, use oral aripiprazole supplementation with the next Aripiprazole lauroxil injection as recommended below.
- Table 3: Recommendation for Concomitant Oral Aripiprazole Supplementation Following Missed Doses(a)
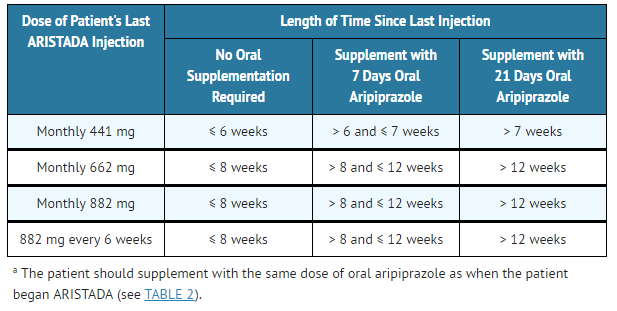
ARISTADA: Aripiprazole lauroxil's Brand name
- Early Dosing
- The recommended Aripiprazole lauroxil dosing interval is either monthly for the 441 mg, 662 mg and 882 mg doses or every 6 weeks for 882 mg dose and should be maintained. In the event of early dosing, an Aripiprazole lauroxil injection should not be given earlier than 14 days after the previous injection.
- Dose Adjustments for CYP450 Considerations
- Refer to the prescribing information for oral aripiprazole for recommendations regarding dosage adjustments due to drug interactions, for the first 21 days when the patient is taking oral aripiprazole concomitantly with the first dose of Aripiprazole lauroxil.
- Table 4: Aripiprazole lauroxil Dose Adjustments with Concomitant CYP450 Modulator Use
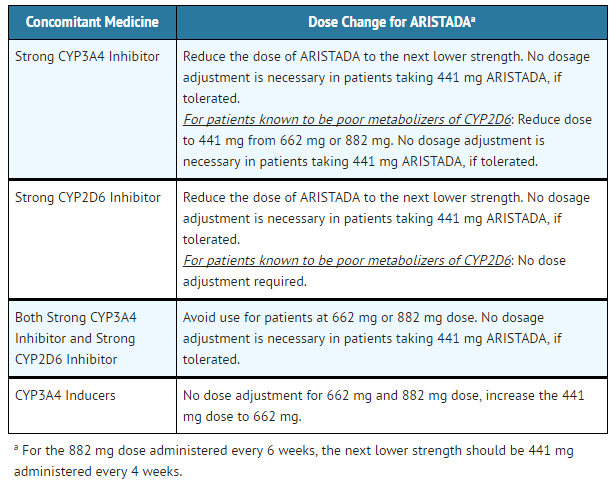
ARISTADA: Aripiprazole lauroxil's Brand name
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aripiprazole lauroxil in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aripiprazole lauroxil in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness of Aripiprazole lauroxil in patients <18 years of age have not been evaluated.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aripiprazole lauroxil in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aripiprazole lauroxil in pediatric patients.
Contraindications
Aripiprazole lauroxil is contraindicated in patients with a known hypersensitivity reaction to aripiprazole. Hypersensitivity reactions have ranged from pruritus/urticaria to anaphylaxis.
Warnings
|
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
See full prescribing information for complete Boxed Warning.
|
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group.
Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Aripiprazole lauroxil is not approved for the treatment of patients with dementia-related psychosis.
Cerebrovascular Adverse Reactions, Including Stroke
In placebo-controlled trials with risperidone, aripiprazole, and olanzapine in elderly patients with dementia, there was a higher incidence of cerebrovascular adverse reactions (cerebrovascular accidents and transient ischemic attacks) including fatalities compared to placebo-treated patients. Aripiprazole lauroxil is not approved for the treatment of patients with dementia-related psychosis.
Neuroleptic Malignant Syndrome
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) may occur in association with antipsychotic drugs, including Aripiprazole lauroxil. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure.
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases in which the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system pathology.
The management of NMS should include: (1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy; (2) intensive symptomatic treatment and medical monitoring; and (3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for uncomplicated NMS.
If a patient appears to require antipsychotic drug treatment after recovery from NMS, reintroduction of drug therapy should be closely monitored, since recurrences of NMS have been reported.
Tardive Dyskinesia
A syndrome of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to predict which patients will develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
The risk of developing tardive dyskinesia and the likelihood that it will become irreversible appear to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase, but the syndrome can develop after relatively brief treatment periods at low doses, although this is uncommon.
There is no known treatment for established tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment itself may suppress (or partially suppress) the signs and symptoms of the syndrome and may thus mask the underlying process. The effect of symptomatic suppression on the long-term course of the syndrome is unknown.
Given these considerations, Aripiprazole lauroxil should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that is known to respond to antipsychotic drugs. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient treated with Aripiprazole lauroxil drug discontinuation should be considered. However, some patients may require treatment with Aripiprazole lauroxil despite the presence of the syndrome.
Metabolic Changes
Atypical antipsychotic drugs have been associated with metabolic changes that include hyperglycemia/diabetes mellitus, dyslipidemia, and weight gain. While all drugs in the class have been shown to produce some metabolic changes, each drug has its own specific risk profile.
- Hyperglycemia, in some cases extreme and associated with ketoacidosis or hyperosmolar coma or death, has been reported in patients treated with atypical antipsychotics. There have been reports of hyperglycemia in patients treated with oral aripiprazole. Assessment of the relationship between atypical antipsychotic use and glucose abnormalities is complicated by the possibility of an increased background risk of diabetes mellitus in patients with schizophrenia and the increasing incidence of diabetes mellitus in the general population. Given these confounders, the relationship between atypical antipsychotic use and hyperglycemia-related adverse events is not completely understood. However, epidemiological studies suggest an increased risk of hyperglycemia-related adverse reactions in patients treated with the atypical antipsychotics.
- Patients with an established diagnosis of diabetes mellitus who are started on atypical antipsychotics should be monitored regularly for worsening of glucose control. Patients with risk factors for diabetes mellitus (e.g., obesity, family history of diabetes) who are starting treatment with atypical antipsychotics should undergo fasting blood glucose testing at the beginning of treatment and periodically during treatment. Any patient treated with atypical antipsychotics should be monitored for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Patients who develop symptoms of hyperglycemia during treatment with atypical antipsychotics should undergo fasting blood glucose testing. In some cases, hyperglycemia has resolved when the atypical antipsychotic was discontinued; however, some patients require continuation of anti-diabetic treatment despite discontinuation of the suspect drug.
- In the long-term, open-label schizophrenia study with Aripiprazole lauroxil, 14% of patients with normal hemoglobin A1c (<5.7%) at baseline developed elevated levels (≥5.7%) post-baseline.
- Undesirable alterations in lipids have been observed in patients treated with atypical antipsychotics.
- In the long-term, open-label schizophrenia study with Aripiprazole lauroxil, shifts in baseline fasting total cholesterol from normal (<200 mg/dL) to high (≥240 mg/dL) were reported in 1% of patients; shifts in baseline fasting LDL cholesterol from normal (<100 mg/dL) to high (≥160 mg/dL) were reported in 1% of patients; and shifts in baseline fasting triglycerides from normal (<150 mg/dL) to high (≥200 mg/dL) were reported in 8% of patients. In the same study, shifts in baseline fasting total cholesterol from borderline (≥ 200 mg/dL and <240 mg/dL) to high (≥240 mg/dL) were reported in 15% of patients; shifts in baseline fasting LDL cholesterol from borderline (≥100 mg/dL and <160 mg/dL) to high (≥160 mg/dL) were reported in 8% of patients; and shifts in baseline fasting triglycerides from borderline (≥150 mg/dL and <200 mg/dL) to high (≥200 mg/dL) were reported in 35% of patients. In addition, the proportion of patients with shifts in fasting HDL cholesterol from normal (≥40 mg/dL) to low (<40 mg/dL) was reported in 15% of patients.
- Weight Gain
- Weight gain has been observed with atypical antipsychotic use. Clinical monitoring of weight is recommended.
- The proportion of adult patients with weight gain ≥7% of body weight is presented in TABLE 6.
- Table 6: Proportion of Adult Patients with Shifts in Weight in the 12-Week, Placebo-Controlled, Fixed-Dose Schizophrenia Trial
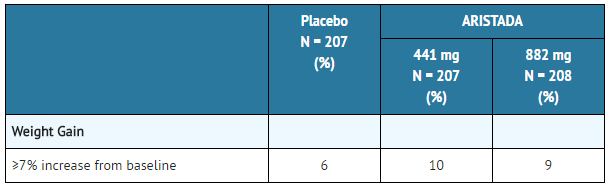
ARISTADA: Aripiprazole lauroxil's Brand name
Pathological Gambling and Other Compulsive Behaviors
Post-marketing case reports suggest that patients can experience intense urges, particularly for gambling, and the inability to control these urges while taking aripiprazole. Other compulsive urges, reported less frequently include: sexual urges, shopping, eating or binge eating, and other impulsive or compulsive behaviors. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to ask patients or their caregivers specifically about the development of new or intense gambling urges, compulsive sexual urges, compulsive shopping, binge or compulsive eating, or other urges while being treated with aripiprazole. It should be noted that impulse-control symptoms can be associated with the underlying disorder. In some cases, although not all, urges were reported to have stopped when the dose was reduced or the medication was discontinued. Compulsive behaviors may result in harm for the patient and others if not recognized. Consider dose reduction or stopping the medication if a patient develops such urges.
Orthostatic Hypotension
Aripiprazole may cause orthostatic hypotension, perhaps due to its α1-adrenergic receptor antagonism. Associated adverse reactions related to orthostatic hypotension can include dizziness, lightheadedness and tachycardia. Generally, these risks are greatest at the beginning of treatment and during dose escalation. Patients at increased risk of these adverse reactions or at increased risk of developing complications from hypotension include those with dehydration, hypovolemia, treatment with antihypertensive medication, history of cardiovascular disease (e.g., heart failure, myocardial infarction, ischemia, or conduction abnormalities), history of cerebrovascular disease, as well as patients who are antipsychotic-naïve. In such patients, consider using a lower starting dose, and monitor orthostatic vital signs.
Orthostatic hypotension was reported for one patient in the Aripiprazole lauroxil 882 mg group (0.5%) and no patients in the Aripiprazole lauroxil 441 mg and placebo groups in the 12-week schizophrenia efficacy study. In the long-term open-label schizophrenia study, orthostatic hypotension was reported for 1 (0.2%) patient treated with Aripiprazole lauroxil. Orthostatic hypotension was defined as a decrease in systolic blood pressure ≥20 mmHg accompanied by an increase in heart rate ≥25 bpm when comparing standing to supine values.
Leukopenia, Neutropenia, and Agranulocytosis
In clinical trials and/or postmarketing experience, events of leukopenia and neutropenia have been reported temporally related to antipsychotic agents. Agranulocytosis has also been reported.
Possible risk factors for leukopenia/neutropenia include pre-existing low white blood cell count (WBC)/absolute neutrophil count (ANC) and history of drug-induced leukopenia/neutropenia. In patients with a history of a clinically significant low WBC/ANC or drug-induced leukopenia/neutropenia, perform a complete blood count (CBC) frequently during the first few months of therapy. In such patients, consider discontinuation of Aripiprazole lauroxil at the first sign of a clinical significant decline in WBC in the absence of other causative factors.
Monitor patients with clinically significant neutropenia for fever or other symptoms or signs of infection and treat promptly if such symptoms or signs occur. Discontinue Aripiprazole lauroxil in patients with severe neutropenia (absolute neutrophil count <1000/mm3) and follow their WBC until recovery.
Seizures
As with other antipsychotic drugs, use Aripiprazole lauroxil cautiously in patients with a history of seizures or with conditions that lower the seizure threshold. Conditions that lower the seizure threshold may be more prevalent in a population of 65 years or older.
Potential for Cognitive and Motor Impairment
Aripiprazole lauroxil, like other antipsychotics, has the potential to impair judgment, thinking or motor skills. Patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that therapy with Aripiprazole lauroxil does not affect them adversely.
Body Temperature Regulation
Disruption of the body's ability to reduce core body temperature has been attributed to antipsychotic agents. Appropriate care is advised when prescribing Aripiprazole lauroxil for patients who will be experiencing conditions which may contribute to an elevation in core body temperature, (e.g., exercising strenuously, exposure to extreme heat, receiving concomitant medication with anticholinergic activity, or being subject to dehydration).
Dysphagia
Esophageal dysmotility and aspiration have been associated with antipsychotic drug use. Aripiprazole lauroxil and other antipsychotic drugs should be used cautiously in patients at risk for aspiration pneumonia.
Adverse Reactions
Clinical Trials Experience
The following are discussed in more details in other sections of the labeling:
- Increased Mortality in Elderly Patients with Dementia-related Psychosis
- Cerebrovascular Adverse Reactions, Including Stroke
- Neuroleptic Malignant Syndrome
- Tardive Dyskinesia
- Metabolic Changes
- Pathological Gambling and Other Compulsive Behaviors
- Orthostatic Hypotension
- Leukopenia, Neutropenia, and Agranulocytosis
- Seizures
- Potential for Cognitive and Motor Impairment
- Body Temperature Regulation
- Dysphagia
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Aripiprazole lauroxil
- Patient Exposure: Aripiprazole lauroxil has been evaluated for safety in 880 adult patients in clinical trials in schizophrenia.
- Commonly Observed Adverse Reactions: The most common adverse reaction (incidence ≥5% and at least twice the rate of placebo in patients treated with Aripiprazole lauroxil) was akathisia.
- Adverse Reactions Occurring at an Incidence of 2% or More in Aripiprazole lauroxil-Treated Patients: Adverse reactions associated with the use of Aripiprazole lauroxil (incidence of 2% or greater, rounded to the nearest percent and Aripiprazole lauroxil incidence greater than placebo) that occurred are shown in TABLE 7.
- Table 7: Adverse Reaction in 2% or More of Aripiprazole lauroxil-Treated Patients and That Occurred at Greater Incidence than in the Placebo-Treated Patients in the 12-Week, Placebo-Controlled, Fixed-Dose Schizophrenia Trial
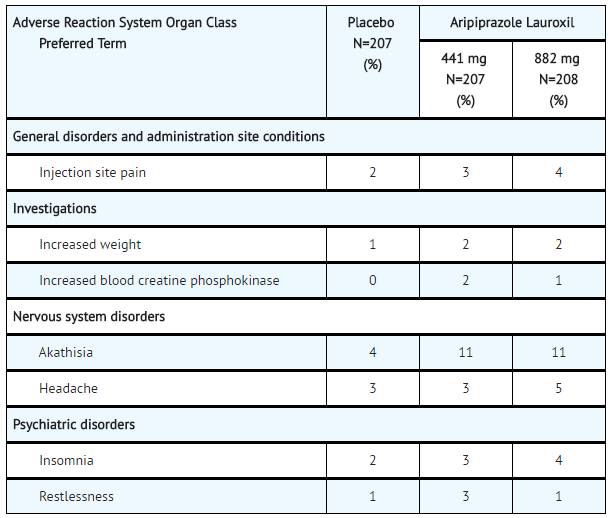
- Injection Site Reactions: Injection site reactions were reported by 4% of patients treated with 441 mg Aripiprazole lauroxil and 5% of patients treated with 882 mg Aripiprazole lauroxil compared to 2% of patients treated with placebo. Most of these were injection site pain (3%, 4% and 2% in the 441 mg Aripiprazole lauroxil, 882 mg Aripiprazole lauroxil and placebo groups, respectively) and most were associated with the first injection, and decreased with each subsequent injection to less than or equal to 1% for both doses of Aripiprazole lauroxil and placebo. Other injection site reactions (induration, swelling and redness) occurred at less than 1%.
- Extrapyramidal Symptoms: In the 12-week schizophrenia efficacy study, for Aripiprazole lauroxil-treated patients, the incidence of other EPS-related events, excluding akathisia and restlessness, was 5% and 7% for patients on 441 mg and 882 mg, respectively, versus 4% for placebo-treated patient (TABLE 8).
- Table 8: Incidence of EPS Compared to Placebo
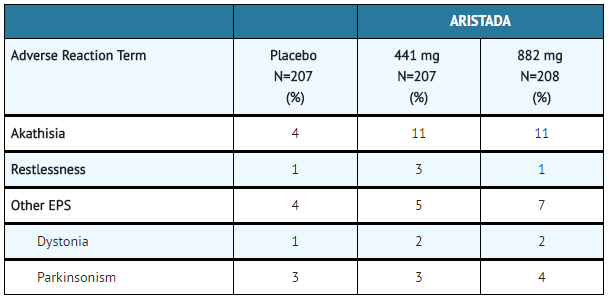
ARISTADA: Aripiprazole lauroxil's Brand name
- Dystonia: Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups.
- Other Adverse Reactions Observed in Clinical Studies: The following listing does not include reactions: 1) already listed in previous tables or elsewhere in labeling, 2) for which a drug cause was remote, 3) which were so general as to be uninformative, 4) which were not considered to have significant clinical implications, or 5) which occurred at a rate equal to or less than placebo.
- Cardiac – angina pectoris, tachycardia, palpitations
- Gastrointestinal disorders – constipation, dry mouth
- General disorders – asthenia
- Musculoskeletal – muscular weakness
- Nervous system disorders – dizziness
Adverse Reactions Reported in Clinical Trials with Oral Aripiprazole
The following is a list of additional adverse reactions that have been reported in clinical trials with oral aripiprazole and not reported above for Aripiprazole lauroxil.
- Blood and Lymphatic System Disorders: thrombocytopenia
- Eye Disorders: photophobia, diplopia
- Gastrointestinal Disorders: gastroesophageal reflux disease
- General Disorders and Administration Site Conditions: peripheral edema, chest pain, face edema
- Immune System Disorders: hypersensitivity
- Injury, Poisoning, and Procedural Complications: fall, heat stroke
- Investigations: weight decreased, hepatic enzyme increased, blood glucose increased, blood lactate dehydrogenase increased, gamma glutamyl transferase increased, blood prolactin increased, blood urea increased, blood creatinine increased, blood bilirubin increased, electrocardiogram QT prolonged, glycosylated hemoglobin increased
- Metabolism and Nutrition Disorders: anorexia, hypokalemia, hyponatremia, hypoglycemia
- Musculoskeletal and Connective Tissue Disorders: muscle tightness, rhabdomyolysis, mobility decreased
- Nervous System Disorders: memory impairment, cogwheel rigidity, hypokinesia, myoclonus, bradykinesia, akinesia, myoclonus, coordination abnormal, speech disorder, choreoathetosis
- Psychiatric Disorders: aggression, loss of libido, delirium, libido increased, anorgasmia, tic, homicidal ideation, catatonia, sleep walking
- Renal and Urinary Disorders: urinary retention, nocturia
- Reproductive System and Breast Disorders: erectile dysfunction, gynaecomastia, menstruation irregular, amenorrhea, breast pain, priapism
- Respiratory, Thoracic, and Mediastinal Disorders: nasal congestion, dyspnea
- Skin and Subcutaneous Tissue Disorders: rash, hyperhidrosis, pruritus, photosensitivity reaction, alopecia, urticaria
- Vascular Disorders: hypotension, hypertension
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of oral aripiprazole. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or to establish a causal relationship to drug exposure: occurrences of allergic reaction (anaphylactic reaction, angioedema, laryngospasm, pruritus/urticaria, or oropharyngeal spasm), pathological gambling, hiccups and blood glucose fluctuation.
Drug Interactions
Drugs Having Clinically Important Interactions with Aripiprazole lauroxil
- Table 9: Clinically Important Drug Interactions with Aripiprazole lauroxil
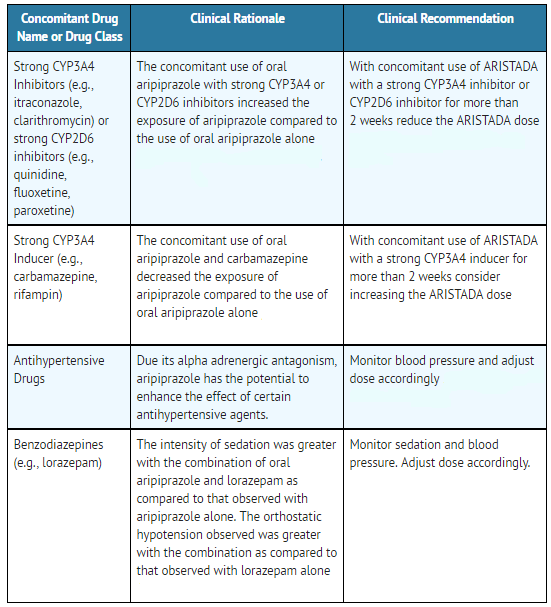
ARISTADA: Aripiprazole lauroxil's Brand name
Drugs Having No Clinically Important Interactions with Aripiprazole lauroxil
Based on pharmacokinetic studies with oral aripiprazole, no dosage adjustment of Aripiprazole lauroxil is required when administered concomitantly with famotidine, valproate, lithium.
In addition, no dosage adjustment is necessary for substrates of CYP2D6 (e.g., dextromethorphan, fluoxetine, paroxetine, or venlafaxine), CYP2C9 (e.g., warfarin), CYP2C19 (e.g., omeprazole, warfarin, escitalopram), or CYP3A4 (e.g., dextromethorphan) when co-administered with Aripiprazole lauroxil. Additionally, no dosage adjustment is necessary for valproate, lithium, lamotrigine, or sertraline when co-administered with Aripiprazole lauroxil.
Use in Specific Populations
Pregnancy
- Pregnancy Exposure Registry
- There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Aripiprazole lauroxil during pregnancy. For more information, contact the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or visit http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/ .
- Risk Summary
- Neonates exposed to antipsychotic drugs during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery. Limited published data on aripiprazole use in pregnant women are not sufficient to inform any drug-associated risks for birth defects or miscarriage. No teratogenicity was observed in animal reproductive studies with intramuscular administration of aripiprazole lauroxil to rats and rabbits during organogenesis at doses up to 6 and 18 times, respectively, the maximum recommended human dose (MRHD) of 882 mg based on body surface area (mg/m2). However, aripiprazole caused developmental toxicity and possible teratogenic effects in rats and rabbits. The background risk of major birth defects and miscarriage for the indicated population are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. Advise pregnant women of the potential risk to a fetus.
- Clinical Considerations
- Fetal/Neonatal Adverse Reactions
- Extrapyramidal and/or withdrawal symptoms, including agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder have been reported in neonates who were exposed to antipsychotic drugs during the third trimester of pregnancy. These symptoms have varied in severity. Monitor neonates for extrapyramidal and/or withdrawal symptoms and manage symptoms appropriately. Some neonates recover within hours or days without specific treatment; others required prolonged hospitalization.
- Data
- Animal Data for Aripiprazole Lauroxil
- Aripiprazole lauroxil did not cause adverse developmental or maternal effects in rats or rabbits when administered intramuscularly during the period of organogenesis at doses of 18, 49, or 144 mg/animal in pregnant rats which are approximately 0.7 to 6 times the maximum recommended human dose (MRHD) of 882 mg on mg/m2 basis, and at doses of 241, 723, and 2893 mg/animal in pregnant rabbits which are approximately 1 to 18 times the MRHD on mg/m2 basis. However, aripiprazole caused developmental toxicity and possible teratogenic effects in rats and rabbits.
- Animal Data for Aripiprazole
- Pregnant rats were treated with oral doses of 3, 10, and 30 mg/kg/day which are approximately 1 to 10 times the oral maximum recommended human dose [MRHD] of 30 mg/day on mg/m2 basis of aripiprazole during the period of organogenesis. Treatment at the highest dose caused a slight prolongation of gestation and delay in fetal development, as evidenced by decreased fetal weight, and undescended testes. Delayed skeletal ossification was observed at 3 and 10 times the oral MRHD on mg/m2 basis.
- At 3 and 10 times the oral MRHD on mg/m2 basis, delivered offspring had decreased body weights. Increased incidences of hepatodiaphragmatic nodules and diaphragmatic hernia were observed in offspring from the highest dose group (the other dose groups were not examined for these findings). A low incidence of diaphragmatic hernia was also seen in the fetuses exposed to the highest dose. Postnatally, delayed vaginal opening was seen at 3 and 10 times the oral MRHD on mg/m2 basis and impaired reproductive performance (decreased fertility rate, corpora lutea, implants, live fetuses, and increased post-implantation loss, likely mediated through effects on female offspring) along with some maternal toxicity were seen at the highest dose; however, there was no evidence to suggest that these developmental effects were secondary to maternal toxicity.
- In pregnant rabbits treated with oral doses of 10, 30, and 100 mg/kg/day which are 2 to 11 times human exposure at the oral MRHD based on AUC and 6 to 65 times the oral MRHD on mg/m2 basis of aripiprazole during the period of organogenesis decreased maternal food consumption and increased abortions were seen at the highest dose as well as increased fetal mortality. Decreased fetal weight and increased incidence of fused sternebrae were observed at 3 and 11 times the oral MRHD based on AUC.
- In rats treated with oral doses of 3, 10, and 30 mg/kg/day which are 1 to 10 times the oral MRHD on mg/m2 basis of aripiprazole perinatally and postnatally (from day 17 of gestation through day 21 postpartum), slight maternal toxicity and slightly prolonged gestation were seen at the highest dose. An increase in stillbirths and decreases in pup weight (persisting into adulthood) and survival were also seen at this dose.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Aripiprazole (intramuscular) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Aripiprazole (intramuscular) during labor and delivery.
Nursing Mothers
Aripiprazole is present in human breast milk; however, there are insufficient data to assess the amount in human milk, the effects on the breastfed infant, or the effects on milk production. The development and health benefits of breastfeeding should be considered along with the mother's clinical need for Aripiprazole lauroxil and any potential adverse effects on the breastfed infant from Aripiprazole lauroxil or from the underlying maternal condition.
Pediatric Use
Safety and effectiveness of Aripiprazole lauroxil in patients <18 years of age have not been evaluated.
Geriatic Use
Safety and effectiveness of Aripiprazole lauroxil in patients >65 years of age have not been evaluated.
Gender
There is no FDA guidance on the use of Aripiprazole (intramuscular) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Aripiprazole (intramuscular) with respect to specific racial populations.
Renal Impairment
No dosage adjustment for Aripiprazole lauroxil is required based on a patient's renal function (mild to severe renal impairment, glomerular filtration rate between 15 and 90 mL/minute).
Hepatic Impairment
No dosage adjustment for Aripiprazole lauroxil is required based on a patient's hepatic function (mild to severe hepatic impairment, Child-Pugh score between 5 and 15).
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Aripiprazole (intramuscular) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Aripiprazole (intramuscular) in patients who are immunocompromised.
Other Specific Populations
No dosage adjustment for Aripiprazole lauroxil is required on the basis of a patient's sex, race, or smoking status.
Administration and Monitoring
Administration
- Instructions for Use
- The kit contains a syringe containing Aripiprazole lauroxil sterile aqueous suspension and 2 or 3 safety needles depending on dose (a 2-inch 20 gauge needle with yellow needle hub, a 1 ½-inch 20 gauge needle with yellow needle hub, and a 1-inch 21 gauge needle with green needle hub (441 mg kit only)) for intramuscular injection. All materials should be stored at room temperature.
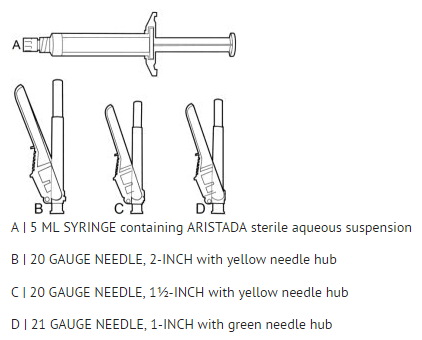
ARISTADA: Aripiprazole lauroxil's Brand name
- 1. TAP and SHAKE the syringe.
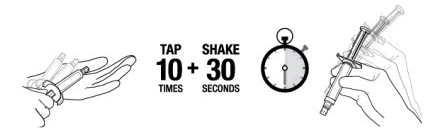
- 1a. Tap the syringe at least 10 times to dislodge any material which may have settled.
- 1b. Shake the syringe vigorously for a minimum of 30 seconds to ensure a uniform suspension. If the syringe is not used within 15 minutes, shake again for 30 seconds.
- 2. SELECT the injection needle.
- 2a. Select injection site.
- 2b. Select needle length based on injection site. For patients with a larger amount of subcutaneous tissue overlaying the injection site muscle, use the longer of the needles provided.
- Table 5: Injection Site and Associated Needle Length
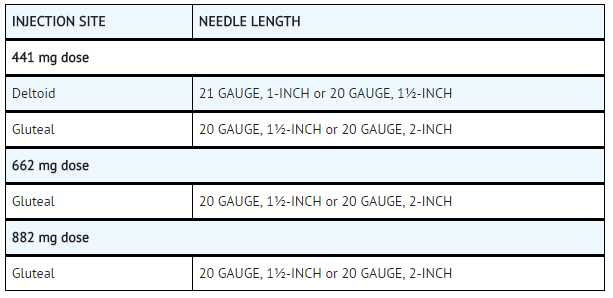
- 3. ATTACH the injection needle.
- Attach the appropriate needle securely with a clockwise twisting motion. Do NOT overtighten. Overtightening could lead to needle hub cracking.
- 4. PRIME the syringe to remove air.
- 4a. Bring the syringe into upright position and tap the syringe to bring air to the top.
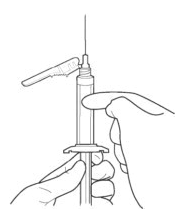
- 4b. Remove air by depressing the plunger rod. A few drops of suspension will be released.
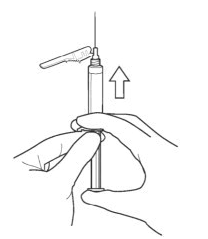
- 5. ADMINISTER the entire content intramuscularly. Do not inject by any other route. Inject in a rapid and continuous manner (less than 10 seconds).
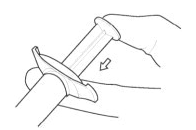
- 6. DISPOSE of the needle. Cover the needle by pressing the safety device. Dispose of used and unused items in a proper waste container.
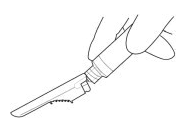
Monitoring
There is limited information regarding Aripiprazole (intramuscular) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Aripiprazole (intramuscular) and IV administrations.
Overdosage
- Human Experience
- The largest known case of acute ingestion with a known outcome involved 1260 mg of oral aripiprazole (42 times the maximum recommended daily dose) in a patient who fully recovered.
- Common adverse reactions (reported in at least 5% of all overdose cases) reported with oral aripiprazole overdosage (alone or in combination with other substances) include vomiting, somnolence, and tremor. Other clinically important signs and symptoms observed in one or more patients with aripiprazole overdoses (alone or with other substances) include acidosis, aggression, aspartate aminotransferase increased, atrial fibrillation, bradycardia, coma, confusional state, convulsion, blood creatine phosphokinase increased, depressed level of consciousness, hypertension, hypokalemia, hypotension, lethargy, loss of consciousness, QRS complex prolonged, QT prolonged, pneumonia aspiration, respiratory arrest, status epilepticus, and tachycardia.
- Management of Overdosage
- In case of overdosage, call the Poison control center immediately at 1-800-222-1222.
Pharmacology
| |
Aripiprazole (intramuscular)
| |
| Systematic (IUPAC) name | |
| [7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-2-oxo-3,4-dihydroquinolin-1-yl]methyl dodecanoate | |
| Identifiers | |
| CAS number | |
| ATC code | ? |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 660.71384 g/mol |
| SMILES | & |
| Synonyms | ALKS-9070, ALKS-9072, RDC-3317 |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | Intramuscular |
Mechanism of Action
Aripiprazole lauroxil is a prodrug of aripiprazole. Following intramuscular injection, Aripiprazole lauroxil is likely converted by enzyme-mediated hydrolysis to N-hydroxymethyl aripiprazole, which is then hydrolyzed to aripiprazole. The mechanism of action of aripiprazole in the body is unknown. However, efficacy could be mediated through a combination of partial agonist activity D2 and 5-HT1A receptors and antagonist activity at 5-HT2A receptors. Actions at receptors other than D2, 5-HT1A, and 5-HT2A could explain some of the adverse reactions of aripiprazole (e.g., the orthostatic hypotension observed with aripiprazole may be explained by its antagonist activity at adrenergic alpha1 receptors).
Structure
Aripiprazole lauroxil is an atypical antipsychotic.
The chemical name of Aripiprazole lauroxil is 7-{4-[4-(2,3-dichlorophenyl)-piperazin-1-yl]butoxy}-2-oxo-3,4-dihydro-2H-quinolin-1-yl)methyl dodecanoate. The empirical formula is C36H51Cl2N3O4 and its molecular weight is 660.7 g/mol. The chemical structure is:
Aripiprazole lauroxil is available as a white to off-white sterile aqueous extended-release suspension for intramuscular injection in the following strengths of aripiprazole lauroxil (and deliverable volumes from a single-use pre-filled syringe): 441 mg (1.6 mL), 662 mg (2.4 mL) and 882 mg (3.2 mL). The inactive ingredients include sorbitan monolaurate (3.8 mg/mL), polysorbate 20 (1.5 mg/mL), sodium chloride (6.1 mg/mL), sodium phosphate dibasic anhydrous, sodium phosphate monobasic and water for injection.
Pharmacodynamics
Aripiprazole exhibits high affinity for dopamine D2 and D3, serotonin 5-HT1A and 5-HT2A receptors (Ki values 0.34 nM, 0.8 nM, 1.7 nM, and 3.4 nM, respectively), moderate affinity for dopamine D4, serotonin 5-HT2C and 5-HT7, alpha1-adrenergic and histamine H1 receptors (Ki values 44 nM, 15 nM, 39 nM, 57 nM, and 61 nM, respectively), and moderate affinity for the serotonin reuptake site (Ki =98 nM). Aripiprazole has no appreciable affinity for cholinergic muscarinic receptors (IC50>1000 nM). Aripiprazole functions as a partial agonist at the dopamine D2 and the serotonin 5-HT1A receptors, and as an antagonist at serotonin 5-HT2A receptor.
Pharmacokinetics
Aripiprazole lauroxil is a prodrug of aripiprazole and its activity in the body is primarily due to aripiprazole, and to a lesser extent dehydro-aripiprazole (major metabolite of aripiprazole), which has been shown to have affinities for D2 receptors similar to aripiprazole and represents 30-40% of the aripiprazole exposure in plasma.
- After single intramuscular injection the appearance of aripiprazole in the systemic circulation starts from 5 to 6 days and continues to be released for an additional 36 days. Aripiprazole concentrations increase with consecutive doses of Aripiprazole lauroxil and reach steady-state following the fourth monthly injection. The concentration-time course of dehydro-aripiprazole followed that of aripiprazole.
- With the addition of oral aripiprazole supplementation for 21 days at the time of the first Aripiprazole lauroxil dose, aripiprazole concentrations reach therapeutic levels within 4 days.
- Based on population pharmacokinetic analysis, the apparent volume of distribution of aripiprazole following intramuscular injection of Aripiprazole lauroxil was 268 L, indicating extensive extravascular distribution following absorption. At therapeutic concentrations, aripiprazole and its major metabolite are greater than 99% bound to serum proteins, primarily to albumin. In healthy human volunteers administered 0.5 mg/day to 30 mg/day oral aripiprazole for 14 days, there was dose-dependent D2 receptor occupancy indicating brain penetration of aripiprazole in humans.
- Administration of 882 mg every 6 weeks results in plasma aripiprazole concentrations that are within the established therapeutic range for 441 to 882 mg monthly.
- Metabolism and Elimination
- The biotransformation of Aripiprazole lauroxil likely involves enzyme-mediated hydrolysis to form N-hydroxymethyl-aripiprazole, which subsequently undergoes water mediated hydrolysis to aripiprazole. Elimination of aripiprazole is mainly through hepatic metabolism involving CYP3A4 and CYP2D6. Dosage adjustments are recommended in CYP2D6 poor metabolizers due to high aripiprazole concentrations.
- The mean aripiprazole terminal elimination half-life ranged from 29.2 days to 34.9 days after every 4-week injection of Aripiprazole lauroxil 441, 662 and 882 mg. The significantly longer aripiprazole apparent half-life compared to oral aripiprazole (mean 75 hours) is attributed to the dissolution and formation rate-limited elimination of aripiprazole following Aripiprazole lauroxil administration.
- Drug Interaction Studies
- No specific drug interaction studies have been performed with Aripiprazole lauroxil. The drug interaction data provided below is obtained from studies with oral aripiprazole.
- Effects of other drugs on the exposures of aripiprazole and dehydro-aripiprazole are summarized in FIGURE 1 and FIGURE 2, respectively. Based on simulation, a 4.5-fold increase in mean Cmax and AUC values at steady-state is expected when extensive metabolizers of CYP2D6 are administered with both strong CYP2D6 and CYP3A4 inhibitors. After oral administration, a 3-fold increase in mean Cmax and AUC values at steady-state is expected in poor metabolizers of CYP2D6 administered with strong CYP3A4 inhibitors.
- Figure 1: The Effects of Other Drugs on Aripiprazole Pharmacokinetics
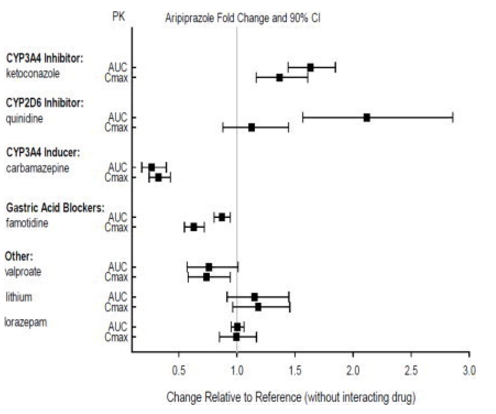
- Figure 2: The Effects of Other Drugs on Dehydro-aripiprazole Pharmacokinetics
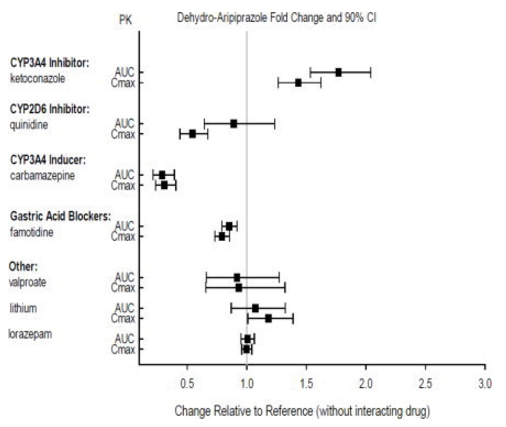
The effects of aripiprazole on the exposures of other drugs are summarized in FIGURE 3.
- Figure 3: The Effects of Oral Aripiprazole on Pharmacokinetics of Other Drugs
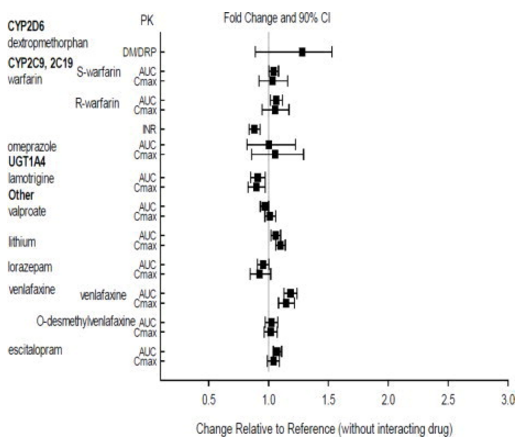
- Specific Population Studies
- A population pharmacokinetic analysis showed no effect of sex, race or smoking on Aripiprazole lauroxil pharmacokinetics.
- Exposures of aripiprazole and dehydro-aripiprazole using oral aripiprazole in specific populations are summarized in FIGURE 4 and FIGURE 5, respectively.
- Figure 4: Effects of Intrinsic Factors on Aripiprazole Pharmacokinetics
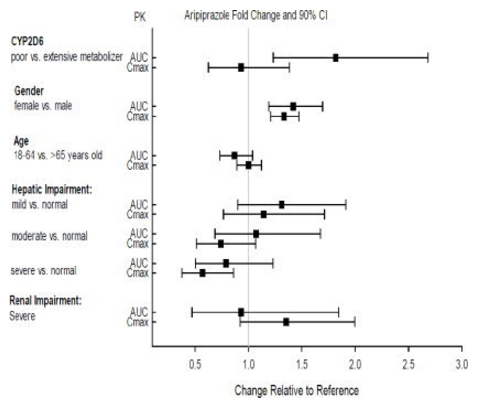
- Figure 5: Effects of Intrinsic Factors on Dehydro-aripiprazole Pharmacokinetics
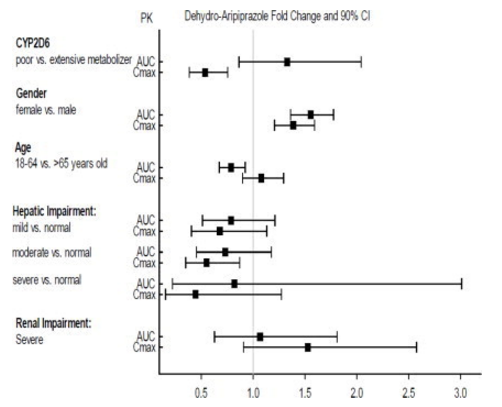
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenesis
- Lifetime carcinogenicity studies have not been conducted with aripiprazole lauroxil.
- Lifetime carcinogenicity studies with oral aripiprazole have been conducted in ICR mice and in Sprague-Dawley (SD) and F344 rats. Aripiprazole was administered for 2 years in the diet at doses of 1, 3, 10, and 30 mg/kg/day to ICR mice and 1, 3, and 10 mg/kg/day to F344 rats (0.2 to 5 times and 0.3 to 3 times the oral maximum recommended human dose [MRHD] of 30 mg/day based on mg/m2, respectively). In addition, SD rats were dosed orally for 2 years at 10, 20, 40, and 60 mg/kg/day (3 to 19 times the oral MRHD based on mg/m2). Aripiprazole did not induce tumors in male mice or rats. In female mice, the incidences of pituitary gland adenomas and mammary gland adenocarcinomas and adenoacanthomas were increased at dietary doses which are 0.1 to 0.9 times human exposure at the oral MRHD based on AUC and 0.5 to 5 times the oral MRHD on mg/m2 basis. In female rats, the incidence of mammary gland fibroadenomas was increased at a dietary dose which is 0.1 times human exposure at the oral MRHD based on AUC and 3 times the oral MRHD on mg/m2 basis; and the incidences of adrenocortical carcinomas and combined adrenocortical adenomas/carcinomas were increased at an oral dose which is 14 times human exposure at oral MRHD based on AUC and 19 times the oral MRHD on mg/m2 basis.
- Proliferative changes in the pituitary and mammary gland of rodents have been observed following chronic administration of other antipsychotic agents and are considered prolactin-mediated. The relevance for human risk of the findings of prolactin-mediated endocrine tumors in rodents is unknown.
- Mutagenesis
- Aripiprazole lauroxil was not mutagenic in the in vitro bacterial reverse mutation assay or clastogenic in the in vitro chromosome aberration assay in human peripheral blood lymphocytes.
- Aripiprazole and its metabolite (2,3-DCPP) were clastogenic in the in vitro chromosome aberration assay in Chinese hamster lung (CHL) cells both in the presence and absence of metabolic activation. The metabolite, 2,3-DCPP, produced increases in numerical aberrations in the in vitro assay in CHL cells in the absence of metabolic activation. A positive response was obtained in the oral in vivo micronucleus assay in mice; however, the response was due to a mechanism not considered relevant to humans.
- Impairment of Fertility
- Animal Data for Aripiprazole Lauroxil
- In a rat fertility study, aripiprazole lauroxil was administered intramuscularly. Males were treated with doses of 18, 49, or 144 mg /animal, which are approximately 0.5 to 4 times the maximum recommended human dose [MRHD] of 882 mg on mg/m2 basis, on Days 1, 21, and 42 prior to and through mating; females were treated at these doses, which are approximately 0.7 to 6 times the MRHD on mg/m2 basis, once 14 days prior to mating.
- In females, persistent diestrus was observed at all doses and the mean number of cycles was significantly decreased at the highest dose together with an increase in the copulatory interval (delay in mating). Additional changes at the high dose included slight increases in corpora lutea and pre-implantation loss, decline in mating, fertility, and fecundity indices in females and lower mating and fertility indices in males.
- Animal Data for Aripiprazole
- Female rats were treated with oral aripiprazole doses of 2, 6, and 20 mg/kg/day, which are 0.6 to 6 times the oral maximum recommended human dose [MRHD] of 30 mg/day on mg/m2 basis, from 2 weeks prior to mating through day 7 of gestation. Estrous cycle irregularities and increased corpora lutea were seen at all doses, but no impairment of fertility was observed. Increased pre-implantation loss was found at 2 and 6 times the oral MRHD on mg/m2 basis and decreased fetal weight was noted at the highest dose which is 6 times the oral MRHD on mg/m2 basis.
- Male rats were treated with oral aripiprazole doses of 20, 40, and 60 mg/kg/day, which are 6 to 19 times the oral MRHD on mg/m2 basis, from 9 weeks prior to and through mating. Disturbances in spermatogenesis at the highest dose and prostate atrophy at the mid and high doses were noted which are 13 and 19 times the oral MRHD on mg/m2 basis, but no impairment of fertility was observed.
Animal Toxicology and/or Pharmacology
Intramuscular administration of aripiprazole lauroxil to rats and dogs was associated with injection site tissue reactions at all doses in rats treated up to 6 months at doses of 15, 29, and 103 mg/animal (which are approximately 0.3 to 2 times and 0.5 to 4 times the maximum recommended human dose [MRHD] of 882 mg on mg/m2 basis in males and females, respectively) and in dogs treated up to 9 months at doses of 147, 662, and 2058 mg/animal (which are approximately 0.5 to 8 times and 0.7 to 10 times the MRHD in males and females, respectively on mg/m2 basis). These injection site tissue reactions consisted of localized granulomatous inflammation and granuloma formation. Transiently impaired limb function and swelling occurred in dogs. The granulomas did not completely resolve 2 months following the last injection in the 6 month rat study and 4 months following the last injection in the 9 month dog study (the low dose groups were not examined for reversibility in these studies).
Orally administered aripiprazole produced retinal degeneration in albino rats in a 26-week chronic toxicity study at a dose of 60 mg/kg, which is 19 times the oral MRHD of 30 mg/day on mg/m2 basis, and in a 2-year carcinogenicity study at doses of 40 mg/kg and 60 mg/kg, which are 13 and 19 times the oral MRHD on mg/m2 basis and 7 to 14 times human exposure at the oral MRHD based on AUC. Evaluation of the retinas of albino mice and of monkeys did not reveal evidence of retinal degeneration. Additional studies to further evaluate the mechanism have not been performed. The relevance of this finding to human risk is unknown.
Clinical Studies
The efficacy of Aripiprazole lauroxil in the treatment of patients with schizophrenia was established, in part, on the basis of efficacy data from trials with the oral formulation of aripiprazole. In addition, the efficacy of Aripiprazole lauroxil was established in a 12-week, randomized, double-blind, placebo controlled, fixed-dose study in adult patients with schizophrenia meeting DSM IV TR criteria [Study 1, n = 622; 207 (Aripiprazole lauroxil 441 mg), 208 (Aripiprazole lauroxil 882 mg), and 207 (placebo)]. After establishing tolerability to oral aripiprazole, patients received oral aripiprazole or placebo daily for the first 3 weeks. The intramuscular (IM) injections were administered on Days 1, 29 and 57.
Efficacy was assessed using Positive and Negative Syndrome Scale (PANSS) and Clinical Global Impression Improvement Scale (CGI-I):
- The PANSS is a 30-item scale that measures positive symptoms of schizophrenia (7 items), negative symptoms of schizophrenia (7 items), and general psychopathology (16 items), each rated on a scale of 1 (absent) to 7 (extreme). Total PANSS scores range from 30 to 210.
- The CGI-I rates improvement in mental illness on a scale of 1 (very much improved) to 7 (very much worse) based on the change from baseline in clinical condition.
Eligible patients were 18 to 70 years of age with PANSS total score of 70 to 120 and a score of ≥4 for at least 2 of the selected Positive Scale items. Patients were also required to have a CGI-S score of ≥4.
The primary efficacy variable was the change from baseline to endpoint (Day 85) in PANSS total score. Statistically significant separation from placebo on PANSS total score change was observed in each Aripiprazole lauroxil dose group (TABLE 10).
- Table 10: Primary Efficacy Results
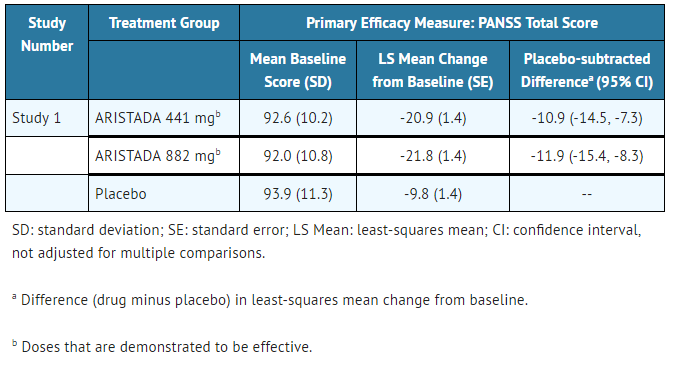
ARISTADA: Aripiprazole lauroxil's Brand name
The visit-wise mean change from baseline on PANSS total score change for each treatment group is shown in FIGURE 6.
- Figure 6: Change from Baseline in PANSS Total Score
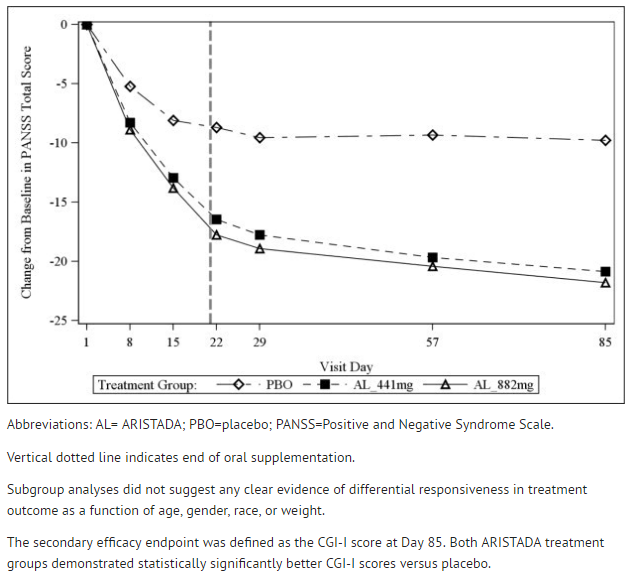
ARISTADA: Aripiprazole lauroxil's Brand name
How Supplied
Aripiprazole lauroxil is a white to off-white aqueous extended-release suspension provided in a single-use pre-filled syringe for intramuscular injection in the deltoid or gluteal muscle at the 441 mg dose strength and in the gluteal muscle at dose strengths of 662 mg and 882 mg.
Aripiprazole lauroxil extended-release injectable suspension is available in strengths of 441 mg in 1.6 mL, 662 mg in 2.4 mL, and 882 mg in 3.2 mL. The kit contains a 5-mL pre-filled syringe containing Aripiprazole lauroxil sterile aqueous suspension and safety needles.
- The 441 mg strength kit (NDC 65757-401-03; light blue label) contains three safety needles; a 1-inch (25 mm) 21 gauge, a 1½-inch (38 mm) 20 gauge, and a 2-inch (50 mm) 20 gauge needle.
- The 662 mg strength kit (NDC 65757-402-03; green label) contains two safety needles; a 1½-inch (38 mm) 20 gauge and a 2-inch (50 mm) 20 gauge needle.
- The 882 mg strength kit (NDC 65757-403-03; burgundy label) contains two safety needles; a 1½-inch (38 mm) 20 gauge and a 2-inch (50 mm) 20 gauge needle.
Aripiprazole lauroxil is available as described in TABLE 11.
- Table 11: Presentations of Aripiprazole lauroxil
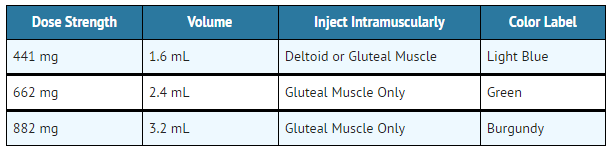
Storage
Store at room temperature 20°C to 25°C (68°F to 77°F) with excursions permitted between 15°C and 30°C (between 59°F and 86°F).
Images
Drug Images
{{#ask: Page Name::Aripiprazole (intramuscular) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel






{{#ask: Label Page::Aripiprazole (intramuscular) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise patients to read FDA-approved patient labeling (MEDICATION GUIDE).
- Pathological Gambling and Other Compulsive Behaviors
- Advise patients and their caregivers of the possibility that they may experience compulsive urges to shop, intense urges to gamble, compulsive sexual urges, binge eating and/or other compulsive urges and the inability to control these urges. In some cases, but not all, the urges were reported to have stopped when the dose was reduced or stopped.
- Counsel patients about a potentially fatal adverse reaction referred to as NMS that has been reported in association with administration of antipsychotic drugs. Advise patients to contact a healthcare provider or report to the emergency room if they experience signs or symptoms of NMS.
- Advise patients that abnormal involuntary movements have been associated with administration of antipsychotic drugs. Counsel patients to notify their healthcare provider if they notice any movements which they cannot control in their face, tongue, or other body part.
- Metabolic Changes (Hyperglycemia and Diabetes Mellitus, Dyslipidemia, and Weight Gain)
- Educate patients about the risk of metabolic changes, how to recognize symptoms of hyperglycemia and diabetes mellitus, and the need for specific monitoring, including blood glucose, lipids, and weight.
- Educate patients about the risk of orthostatic hypotension (symptoms include feeling dizzy or lightheaded upon standing), particularly at the time of initiating treatment, re-initiating treatment, or increasing the dose.
- Interference with Cognitive and Motor Performance
- Because Aripiprazole lauroxil may have the potential to impair judgment, thinking or motor skills, instruct patients to be cautious about operating hazardous machinery, including automobiles, until they are reasonably certain that Aripiprazole lauroxil therapy does not affect them adversely.
- Heat Exposure and Dehydration
- Advise patients regarding appropriate care in avoiding overheating and dehydration.
- Concomitant Medication
- Advise patients to inform their physicians if they are taking, or plan to take, any prescription or over-the-counter drugs, since there is a potential for interactions.
- Pregnancy
- Advise patients that Aripiprazole lauroxil may cause extrapyramidal and/or withdrawal symptoms in a neonate and to notify their healthcare provider with a known or suspected pregnancy. Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Aripiprazole lauroxil during pregnancy.

Precautions with Alcohol
Alcohol-Aripiprazole (intramuscular) interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
ARISTADA®
Look-Alike Drug Names
There is limited information regarding Aripiprazole (intramuscular) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.