Basal cell carcinoma pathophysiology: Difference between revisions
No edit summary |
|||
| (140 intermediate revisions by 12 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | |||
{{Basal cell carcinoma}} | {{Basal cell carcinoma}} | ||
{{CMG}} | {{CMG}};{{AE}}{{M.N}} | ||
==Overview== | |||
Basal cell carcinoma is one of the most common [[skin cancers]]. It is commonly known as [[rodent ulcer]] due to its distinct [[Morphology (biology)|morphology]] characterized by pearly pink [[nodules]] with [[telangiectasias]], rolled borders, and central crusting with or without an [[Ulceration|ulcerating]] [[lesion]]. The majority common [[Causes|cause]] for the [[development]] of the basal cell carcinoma involves [[radiation exposure]] and [[mutations]] that involve many [[genes]] including sonic [[Hedgehog (cell signaling)|hedgehog]] [[gene]], [[PTCH1]] [[gene]], and other [[Gain-of-function mutation|gain-of-function mutations]] which further depend on the subtypes such as [[nodular]], [[superficial]], Infundibulocystic, [[fibroepithelial]], morpheaform, infiltrative, micronodular, and basosquamous basal cell carcinomas. | |||
==Pathophysiology== | ==Pathophysiology== | ||
[[ | ===Pathogenesis=== | ||
The exact pathogenesis of basal cell carcinoma is not completely understood | |||
===Genetics=== | |||
The development of basal cell carcinoma is the result of multiple genetic mutations such as sonic hedgehog pathway mutations, and PTCH1 gene mutations | |||
*A [[number]] of aberrations involving the [[sonic hedgehog]] [[signaling pathway]](SHH) are noted.<ref name="pmid24587976">{{cite journal |vauthors=Mohan SV, Chang AL |title=Advanced Basal Cell Carcinoma: Epidemiology and Therapeutic Innovations |journal=Curr Dermatol Rep |volume=3 |issue= |pages=40–45 |date=2014 |pmid=24587976 |pmc=3931971 |doi=10.1007/s13671-014-0069-y |url=}}</ref><ref name="pmid29165358">{{cite journal |vauthors=Pellegrini C, Maturo MG, Di Nardo L, Ciciarelli V, Gutiérrez García-Rodrigo C, Fargnoli MC |title=Understanding the Molecular Genetics of Basal Cell Carcinoma |journal=Int J Mol Sci |volume=18 |issue=11 |pages= |date=November 2017 |pmid=29165358 |pmc=5713451 |doi=10.3390/ijms18112485 |url=}}</ref><ref name="pmid30405815">{{cite journal |vauthors=Yunoki T, Tabuchi Y, Hirano T, Miwa S, Imura J, Hayashi A |title=Gene networks in basal cell carcinoma of the eyelid, analyzed using gene expression profiling |journal=Oncol Lett |volume=16 |issue=5 |pages=6729–6734 |date=November 2018 |pmid=30405815 |pmc=6202553 |doi=10.3892/ol.2018.9484 |url=}}</ref><ref name="pmid26029015">{{cite journal |vauthors=Marzuka AG, Book SE |title=Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management |journal=Yale J Biol Med |volume=88 |issue=2 |pages=167–79 |date=June 2015 |pmid=26029015 |pmc=4445438 |doi= |url=}}</ref> | |||
*This pathway is [[vital]] for the [[Regulation of gene expression|regulation]] of [[cell growth]], and [[differentiation]] and loss of [[inhibition]] of this pathway is associated with [[development]] of basal cell cancer. | |||
*The majority of [[mutations]] in sporadic basal cell carcinoma and [[basal cell nevus syndrome]](BCNS) [[patients]] occur in [[PTCH1]] [[gene]], a [[protein]] that inhibits smoothened [[gene]] (SMO). | |||
*The [[second]] most common [[mutation]] in sporadic basal cell carcinoma and [[basal cell nevus syndrome]](BCNS) [[patients]] are [[Gain-of-function mutation|gain-of-function]] [[mutations]] of the smoothened [[gene]] (SMO). | |||
*Loss of [[PTCH1]] results in the failure of Smoothened [[inhibition]], subsequently leading to increases in [[GLI1]] levels, changes in [[transcription]], and subsequent [[tumorigenesis]]. | |||
*[[Gain-of-function mutation|Gain-of-function]] smoothened(SMO) [[mutations]] also leads to increased [[GLI1]] levels and [[tumorigenesis]] | |||
{{Family tree/start}} | |||
{{Family tree | | | | A01 | | | | A02 | |A01= Loss of PTCH1| A02= Gain of function SMO}} | |||
{{Family tree | | | | |!| | | | | |!| }} | |||
{{Family tree | | | | |!| | | | | |!| }} | |||
{{Family tree | | | | B01 | | | | B02 | |B01= Lack of SMO inhibition| B02= Activation of<br>SMO-GLI signaling}} | |||
{{Family tree | | | | |!| | | | | |!|}} | |||
{{Family tree | | | | |!| | | | | |!|}} | |||
{{Family tree | | | | C01 |-|-|-|-|'|C01= ↑GLI1 levels }} | |||
{{Family tree | | | | |!| | | | | }} | |||
{{Family tree | | | | |!| | | | | }} | |||
{{Family tree | | | | D01 | | | |D01= Changes in transcription}} | |||
{{Family tree | | | | |!| | | | | }} | |||
{{Family tree | | | | |!| | | | | }} | |||
{{Family tree | | | | E01 | | | |E01= Tumorigenesis}} | |||
{{Family tree/end}} | |||
[[File:Soinic hedgehog pathway signalling.jpg|thumb|500px|none|Sonic hedgehog signaling pathway. SHH ligand binds to and inhibits the PTCH transmembrane protein. The inhibition of PTCH relieves suppression of / (Smoothened), which then activates the GLI transcription factors. The GLI proteins translocate from the cytoplasm to the nucleus, where they drive gene transcription. (Courtesy of Alexander G. Marzuka, MD),https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4445438/]] | |||
Other Genetic Changes: | |||
*Point mutations in the TP53 gene, the tumor supressor gene are the second most common genetic alteration noticed in BCCs | |||
*Some mutations in the CDKN2A locus and in ras gene family (H-ras, K-ras, and N-ras) are also identified in a smaller proportion of sporadic BCCs | |||
While [[DNA repair]] | ===Enviromental Exposure=== | ||
*Basal cell carcinomas develop in the [[basal cell layer]] of the [[skin]].<ref name="pmid28954101">{{cite journal |vauthors=Montagna E, Lopes OS |title=Molecular basis of basal cell carcinoma |journal=An Bras Dermatol |volume=92 |issue=4 |pages=517–520 |date=2017 |pmid=28954101 |pmc=5595599 |doi=10.1590/abd1806-4841.20176544 |url=}}</ref> | |||
*Cumulative [[DNA damage]] caused by [[chronic]] [[sunlight]] exposure results in [[DNA mutations]] that predispose to the [[development]] of basal cell carcinoma. | |||
*While [[DNA repair]] eliminates most [[Ultraviolet|UV-]]<nowiki/>induced damage, not all cross-links are excised, which eventually results in [[mutation]]s. | |||
*Apart from the [[mutagenesis]], [[sunlight]] [[depresses]] the local [[immune system]], possibly decreasing [[immune]] surveillance for [[new]] [[Tumor cell|tumor cells]]. | |||
===Gross and microscopic pathology=== | |||
*On gross and microscopic histopathological analysis the characteristic findings of basal cell carcinoma are described as below: | |||
*Basal cell carcinoma [[pathological]] features mainly depend upon the subtype. The following table summarizes them:<ref name="CameronLee2019">{{cite journal|last1=Cameron|first1=Michael C.|last2=Lee|first2=Erica|last3=Hibler|first3=Brian P.|last4=Barker|first4=Christopher A.|last5=Mori|first5=Shoko|last6=Cordova|first6=Miguel|last7=Nehal|first7=Kishwer S.|last8=Rossi|first8=Anthony M.|title=Basal cell carcinoma|journal=Journal of the American Academy of Dermatology|volume=80|issue=2|year=2019|pages=303–317|issn=01909622|doi=10.1016/j.jaad.2018.03.060}}</ref><ref name="pmid25134314">{{cite journal |vauthors=Sehgal VN, Chatterjee K, Pandhi D, Khurana A |title=Basal cell carcinoma: pathophysiology |journal=Skinmed |volume=12 |issue=3 |pages=176–81 |date=2014 |pmid=25134314 |doi= |url=}}</ref> | |||
{| class="wikitable" | |||
|- | |||
| rowspan="2" align="center" style="background: #4479BA;" | {{fontcolor|#FFF|'''Subtypes of BCC'''}} | |||
| colspan="2" align="center" style="background: #4479BA;" | {{fontcolor|#FFF|'''Gross features'''}} | |||
| colspan="2" align="center" style="background: #4479BA;" | {{fontcolor|#FFF|''' Microscopic features'''}} | |||
|- | |||
| align="center" style="background: #4479BA;" | {{fontcolor|#FFF|'''Findings'''}} | |||
| align="center" style="background: #4479BA;" | {{fontcolor|#FFF|'''Images'''}} | |||
| align="center" style="background: #4479BA;" | {{fontcolor|#FFF|'''Findings'''}} | |||
| align="center" style="background: #4479BA;" | {{fontcolor|#FFF|'''Images'''}} | |||
|- | |||
| [[Nodular]] | |||
| | |||
*Shiny, pearly [[papule]] or [[nodule]] with a smooth surface | |||
*Rolled borders and [[telangiectasias]] | |||
*Mostly seen on the [[head]] and [[neck]] | |||
| | |||
[[File:BCC Nodular type.jpg|thumb|center|150px|M. Sand, D. Sand, C. Thrandorf, V. Paech, P. Altmeyer, F. G. Bechara [CC BY 2.0 (https://creativecommons.org/licenses/by/2.0)], via Wikimedia Commons]] | |||
| | |||
*[[Discrete distribution|Discrete]] nests of [[malignant]] basaloid [[cells]] in the [[dermis]] | |||
*Peripheral palisading | |||
*Mucoid [[stroma]] containing plump [[spindle cells]] | |||
| | |||
[[File:Basal cell carcinoma histopathology (3).jpg|thumb|center|200px|No machine-readable author provided. KGH assumed (based on copyright claims). [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0/)], via Wikimedia Commons,https://upload.wikimedia.org/wikipedia/commons/b/b6/Basal_cell_carcinoma_histopathology_%283%29.jpg]] | |||
|- | |||
| [[Superficial]] | |||
| | |||
*Well-circumscribed | |||
*[[Erythematous]] thin [[plaque]] or patch with scale | |||
*[[Central]] clearing and thin rolled borders | |||
*Most common on the [[trunk]] | |||
| | |||
[[File:Basal cell carcinoma, superficial.jpg|thumb|center|200px|Kelly Nelson (Photographer) [Public domain], via Wikimedia Commons,https://upload.wikimedia.org/wikipedia/commons/3/32/Basal_cell_carcinoma%2C_superficial.jpg]] | |||
| | |||
*Multiple [[lobular]] foci of basaloid palisading [[keratinocyte]] [[tumors]] | |||
*These are usually attached [[Superficial|superficially]] to the [[epidermis]] with a myxoid [[stroma]] and band-like [[Lichen|lichenoid]] infiltrate | |||
| | |||
[[File:Basal cell carcinoma histopathology (2).jpg|center|thumb|200px|machine-readable author provided. KGH assumed (based on copyright claims). [CC BY-SA 3.0 (http://creativecommons.org/licenses/by-sa/3.0/)], via Wikimedia Commons]] | |||
|- | |||
| Infundibulocystic | |||
| | |||
*Well-circumscribed pearly [[papule]] | |||
*Most common on the [[head]] and [[neck]] region | |||
| | |||
| | |||
*Well-circumscribed | |||
*[[Anastomosing]] strands of basaloid [[Cells (biology)|cells]] and scattered [[infundibulum]]-like [[cystic]] structures | |||
| | |||
|- | |||
| Fibroepithelial | |||
| | |||
*[[Skin]]-colored/[[erythematous]] | |||
*[[Sessile]] [[plaque]]/[[pedunculated]] papulonodule | |||
*They have a predilection for [[trunk]] region | |||
| | |||
| | |||
*Multiple collections of delicate strands of [[epidermal]] basaloid [[keratinocytes]] | |||
*These are usually arranged in a [[reticular]] pattern within a [[spindle cell]] [[stroma]] | |||
| | |||
|- | |||
| Morpheaform | |||
| | |||
*Infiltrated [[plaque]] with poorly defined borders and shiny [[Surface area|surface]] | |||
*Most common on [[head]] and [[neck]] region | |||
| | |||
[[File:PMC3339125 JCAS-5-3-g004.png|thumb|center|150px|Dermatology Centre, Salford Royal Hospital, NHS Foundation Trust, Stott Lane, Salford M6 8HD, UK.]] | |||
| | |||
*Thin cords of basaloid [[Cells (biology)|cells]] surrounded by a sclerotic [[collagenous]] [[stroma]] | |||
*Absent peripheral palisading and [[stromal]] [[cleft]] formation | |||
*Positive staining of [[tumor]] [[stroma]] with [[smooth muscle]] [[alpha-actin]] | |||
| | |||
[[File:PMC4513413 IDOJ-6-286-g002.png|thumb|center|200px|Department of Pathology, Columbia University Medical Center, New York, USA]] | |||
|- | |||
| Infiltrative | |||
| | |||
*Poorly defined | |||
*[[Induration|Indurated]], flat or depressed [[plaque]] with white, yellow, or pale pink color | |||
*They may have overlying crusts, erosions, [[ulcerations]], or [[papules]] | |||
| | |||
[[File:Basal cell carcinoma (1).jpg|thumb|center|200px|Kelly Nelson (Photographer) [Public domain], via Wikimedia Commons,https://upload.wikimedia.org/wikipedia/commons/9/9b/Basal_cell_carcinoma_%281%29.jpg,]] | |||
| | |||
*Thin cords with angulated ends of few basaloid [[keratinocytes]] | |||
*Usually embedded in a classic [[mucinous]]/myxoid [[stroma]] | |||
| | |||
|- | |||
| Micronodular | |||
| | |||
*[[Erythematous]] [[macule]] or thin [[papule]]/[[plaque]] | |||
| | |||
| | |||
*Multiple small aggregates of basaloid [[Cells (biology)|cells]] within the [[dermis]], with subtle peripheral palisading and retraction artifact | |||
| | |||
|- | |||
| Basosquamous | |||
| | |||
*Majority found on the [[head]] and [[neck]] | |||
| | |||
| | |||
*Well-defined [[nodular]] or [[superficial]] BCC component overlying an [[invasive]] front showing basal cell carcinoma and [[squamous cell carcinoma]] [[histologic]] feature | |||
| | |||
|} | |||
===Video=== | |||
{{#ev:youtube|JnJXrFnvOKs}} | |||
==References== | ==References== | ||
{{Reflist|2}} | |||
{{WikiDoc Help Menu}} | {{WikiDoc Help Menu}} | ||
{{WikiDoc Sources}} | {{WikiDoc Sources}} | ||
[[Category:Up-To-Date]] | |||
[[Category: | [[Category:Oncology]] | ||
[[Category:Medicine]] | |||
[[Category:Dermatology]] | [[Category:Dermatology]] | ||
[[Category: | [[Category:Surgery]] | ||
Latest revision as of 18:25, 4 April 2019
|
Basal cell carcinoma Microchapters |
|
Diagnosis |
|---|
|
Case Studies |
|
Basal cell carcinoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Basal cell carcinoma pathophysiology |
|
Risk calculators and risk factors for Basal cell carcinoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Maneesha Nandimandalam, M.B.B.S.[2]
Overview
Basal cell carcinoma is one of the most common skin cancers. It is commonly known as rodent ulcer due to its distinct morphology characterized by pearly pink nodules with telangiectasias, rolled borders, and central crusting with or without an ulcerating lesion. The majority common cause for the development of the basal cell carcinoma involves radiation exposure and mutations that involve many genes including sonic hedgehog gene, PTCH1 gene, and other gain-of-function mutations which further depend on the subtypes such as nodular, superficial, Infundibulocystic, fibroepithelial, morpheaform, infiltrative, micronodular, and basosquamous basal cell carcinomas.
Pathophysiology
Pathogenesis
The exact pathogenesis of basal cell carcinoma is not completely understood
Genetics
The development of basal cell carcinoma is the result of multiple genetic mutations such as sonic hedgehog pathway mutations, and PTCH1 gene mutations
- A number of aberrations involving the sonic hedgehog signaling pathway(SHH) are noted.[1][2][3][4]
- This pathway is vital for the regulation of cell growth, and differentiation and loss of inhibition of this pathway is associated with development of basal cell cancer.
- The majority of mutations in sporadic basal cell carcinoma and basal cell nevus syndrome(BCNS) patients occur in PTCH1 gene, a protein that inhibits smoothened gene (SMO).
- The second most common mutation in sporadic basal cell carcinoma and basal cell nevus syndrome(BCNS) patients are gain-of-function mutations of the smoothened gene (SMO).
- Loss of PTCH1 results in the failure of Smoothened inhibition, subsequently leading to increases in GLI1 levels, changes in transcription, and subsequent tumorigenesis.
- Gain-of-function smoothened(SMO) mutations also leads to increased GLI1 levels and tumorigenesis
| Loss of PTCH1 | Gain of function SMO | ||||||||||||||||||||||||
| Lack of SMO inhibition | Activation of SMO-GLI signaling | ||||||||||||||||||||||||
| ↑GLI1 levels | |||||||||||||||||||||||||
| Changes in transcription | |||||||||||||||||||||||||
| Tumorigenesis | |||||||||||||||||||||||||

Other Genetic Changes:
- Point mutations in the TP53 gene, the tumor supressor gene are the second most common genetic alteration noticed in BCCs
- Some mutations in the CDKN2A locus and in ras gene family (H-ras, K-ras, and N-ras) are also identified in a smaller proportion of sporadic BCCs
Enviromental Exposure
- Basal cell carcinomas develop in the basal cell layer of the skin.[5]
- Cumulative DNA damage caused by chronic sunlight exposure results in DNA mutations that predispose to the development of basal cell carcinoma.
- While DNA repair eliminates most UV-induced damage, not all cross-links are excised, which eventually results in mutations.
- Apart from the mutagenesis, sunlight depresses the local immune system, possibly decreasing immune surveillance for new tumor cells.
Gross and microscopic pathology
- On gross and microscopic histopathological analysis the characteristic findings of basal cell carcinoma are described as below:
- Basal cell carcinoma pathological features mainly depend upon the subtype. The following table summarizes them:[6][7]
| Subtypes of BCC | Gross features | Microscopic features | ||
| Findings | Images | Findings | Images | |
| Nodular |
|
 |
 | |
| Superficial |
|
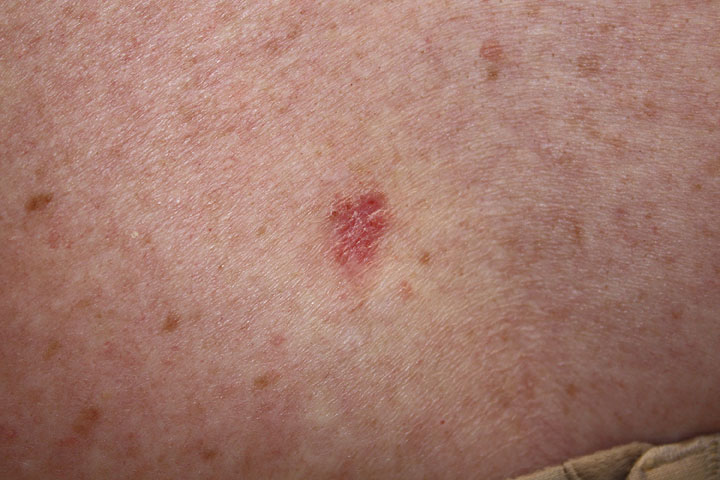 |
|
 |
| Infundibulocystic |
|
|||
| Fibroepithelial |
|
|
||
| Morpheaform |
 |
|
 | |
| Infiltrative |
|
 |
|
|
| Micronodular |
|
|||
| Basosquamous |
|
|||
Video
{{#ev:youtube|JnJXrFnvOKs}}
References
- ↑ Mohan SV, Chang AL (2014). "Advanced Basal Cell Carcinoma: Epidemiology and Therapeutic Innovations". Curr Dermatol Rep. 3: 40–45. doi:10.1007/s13671-014-0069-y. PMC 3931971. PMID 24587976.
- ↑ Pellegrini C, Maturo MG, Di Nardo L, Ciciarelli V, Gutiérrez García-Rodrigo C, Fargnoli MC (November 2017). "Understanding the Molecular Genetics of Basal Cell Carcinoma". Int J Mol Sci. 18 (11). doi:10.3390/ijms18112485. PMC 5713451. PMID 29165358.
- ↑ Yunoki T, Tabuchi Y, Hirano T, Miwa S, Imura J, Hayashi A (November 2018). "Gene networks in basal cell carcinoma of the eyelid, analyzed using gene expression profiling". Oncol Lett. 16 (5): 6729–6734. doi:10.3892/ol.2018.9484. PMC 6202553. PMID 30405815.
- ↑ Marzuka AG, Book SE (June 2015). "Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management". Yale J Biol Med. 88 (2): 167–79. PMC 4445438. PMID 26029015.
- ↑ Montagna E, Lopes OS (2017). "Molecular basis of basal cell carcinoma". An Bras Dermatol. 92 (4): 517–520. doi:10.1590/abd1806-4841.20176544. PMC 5595599. PMID 28954101.
- ↑ Cameron, Michael C.; Lee, Erica; Hibler, Brian P.; Barker, Christopher A.; Mori, Shoko; Cordova, Miguel; Nehal, Kishwer S.; Rossi, Anthony M. (2019). "Basal cell carcinoma". Journal of the American Academy of Dermatology. 80 (2): 303–317. doi:10.1016/j.jaad.2018.03.060. ISSN 0190-9622.
- ↑ Sehgal VN, Chatterjee K, Pandhi D, Khurana A (2014). "Basal cell carcinoma: pathophysiology". Skinmed. 12 (3): 176–81. PMID 25134314.