Pyruvic acid
|
WikiDoc Resources for Pyruvic acid |
|
Articles |
|---|
|
Most recent articles on Pyruvic acid Most cited articles on Pyruvic acid |
|
Media |
|
Powerpoint slides on Pyruvic acid |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Pyruvic acid at Clinical Trials.gov Clinical Trials on Pyruvic acid at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Pyruvic acid
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Pyruvic acid Discussion groups on Pyruvic acid Patient Handouts on Pyruvic acid Directions to Hospitals Treating Pyruvic acid Risk calculators and risk factors for Pyruvic acid
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Pyruvic acid |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
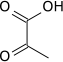
Pyruvic acid (CH3COCO2H) is an alpha-keto acid. Pyruvate plays an important role in biochemical processes. The carboxylate anion of pyruvic acid is known as pyruvate.
Chemistry
Pyruvic acid is a colorless liquid with a smell similar to that of acetic acid. It is miscible with water, and soluble in ethanol and diethyl ether. In the laboratory, pyruvic acid may be prepared by heating a mixture of tartaric acid and potassium hydrogen sulfate, or by the hydrolysis of acetyl cyanide, formed by reaction of acetyl chloride with potassium cyanide:
- CH3COCl + KCN → CH3COCN
- CH3COCN → CH3COCOOH
Biochemical role
Pyruvate is an important chemical compound in biochemistry. It is the output of the aerobic metabolism of glucose known as glycolysis. One molecule of glucose breaks down into two molecules of pyruvic acid, which are then used to provide further energy, in one of two ways. Pyruvic acid is converted into acetyl-coenzyme A, which is the main input for a series of reactions known as the Krebs cycle. Pyruvate is also converted to oxaloacetate by an anaplerotic reaction which replenishes Krebs cycle intermediates; alternatively, the oxaloacetate is used for gluconeogenesis. These reactions are named after Hans Adolf Krebs, the biochemist awarded the 1953 Nobel Prize for physiology, jointly with Fritz Lipmann, for research into metabolic processes. The cycle is also called the citric acid cycle, because citric acid is one of the intermediate compounds formed during the reactions.
If insufficient oxygen is available, the acid is broken down anaerobically, creating lactic acid in animals and ethanol in plants. Pyruvate from glycolysis is converted by anaerobic respiration to lactate using the enzyme lactate dehydrogenase and the coenzyme NADH in lactate fermentation, or to acetaldehyde and then to ethanol in alcoholic fermentation.
Pyruvic acid is a key intersection in the network of metabolic pathways. Pyruvic acid can be converted to carbohydrates via gluconeogenesis, to fatty acids or energy through acetyl-CoA, to the amino acid alanine and to ethanol. Therefore it unites several key metabolic processes.
The pyruvic acid derivative bromopyruvic acid is being studied for potential cancer treatment applications, by Young Hee Ko at Johns Hopkins University and others in ways that would support the Warburg hypothesis on the cause(s) of cancer.
Pyruvate production by glycolysis
In glycolysis, phosphoenolpyruvate (PEP) is converted to pyruvate by pyruvate kinase. This reaction is strongly exergonic and irreversible; in gluconeogenesis it takes two enzymes, pyruvate carboxylase and PEP carboxykinase to catalyze the reverse transformation of pyruvate to PEP. The arrow indicating a reverse reaction in the Figure below is incorrect.
| phosphoenolpyruvate | {{{forward_enzyme}}} | pyruvate | |
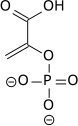
|
| ||
| {{{minor_forward_substrate(s)}}} | {{{minor_forward_product(s)}}} | ||
| [[image:Biochem_reaction_arrow_{{{reaction_direction_(forward/reversible/reverse)}}}_NNYY_horiz_med.svg|75px]] | |||
| ADP | ATP | ||
| Pyruvate kinase | |||
Compound C00074 at KEGG Pathway Database. Enzyme 2.7.1.40 at KEGG Pathway Database. Compound C00022 at KEGG Pathway Database.
Pyruvate decarboxylation to acetyl CoA
Pyruvate decarboxylation by the pyruvate dehydrogenase complex produces acetyl-CoA.
| pyruvate | {{{forward_enzyme}}} | acetyl-CoA | |

|
|||
| {{{minor_forward_substrate(s)}}} | {{{minor_forward_product(s)}}} | ||
| [[image:Biochem_reaction_arrow_{{{reaction_direction_(forward/reversible/reverse)}}}_NNNN_horiz_med.svg|75px]] | |||
Note that decarboxylation is only one of several possible reactions for pyruvate.
Role in the origin of life
Current evolutionary theory on the origin of life posits that the first organisms were anaerobic because the atmosphere of prebiotic Earth was almost devoid of oxygen. As such, requisite biochemical materials must have preceded life and recent experiments indicate that pyruvate can be synthesized abiotically. In vitro, iron sulfide at sufficient pressure and temperature catalyzes the formation of pyruvic acid. Thus, argues Günter Wächtershäuser, the mixing of iron-rich crust with hydrothermal vent fluid is suspected of providing the fertile basis for the formation of life.
External links
ATP
ADP
ATP
ADP
+ +
NAD++ Pi
NADH + H+
NAD++ Pi
NADH + H+ H2O
H2O ADP
ATP
2 × Pyruvate 2 × File:Pyruvat.svg
|
References
- George D. Cody, Nabil Z. Boctor, Timothy R. Filley, Robert M. Hazen, James H. Scott, Anurag Sharma, Hatten S. Yoder Jr., "Primordial Carbonylated Iron-Sulfur Compounds and the Synthesis of Pyruvate," Science, 289 (5483) (25 August 2000) pp. 1337 - 1340. [2]
cs:Kyselina pyrohroznová da:Pyruvat de:Brenztraubensäure de:Pyruvate id:Asam piruvat it:acido piruvico lv:Pirovīnogskābe lb:Pyruvat hu:Piroszőlősav nl:Pyrodruivenzuur fi:Pyruvaatti sv:Pyrodruvsyra