Teeth
Editor-In-Chief: Henry A. Hoff
Teeth are found in the mouths of humans and many other animals. The word teeth is the plural of the word tooth. Teeth are of various shapes, sizes and lengths.
Normally adult humans have 32 teeth in their mouth which help chew food.
Teeth have a natural coating which is called enamel.
Necessary care needs be excercised while cleaning or brushing them. Teeth in good condition have aesthetic appeal that attracts and pleases viewers.
Theoretical teeth
Def. a "hard, calcareous structure present in the mouth of many vertebrate animals, generally used for eating"[1] is called a tooth.
The pattern of incisors, canines, premolars and molars is found only in mammals, and to varying extents, in their evolutionary ancestors, but the numbers of these types of teeth vary greatly between species; zoologists use a standardised dental formula to describe the precise pattern in any given group.[2]
The image on the right is a model of a human molar-like tooth. Its components are labeled:
- Tooth:
- Enamel
- Dentin
- Dental pulp:
- cameral pulp
- root pulp
- Cementum
- Crown
- Cusp
- Sulcus
- Cementoenamel junction or Neck
- Root
- Furcation
- Root apex
- Apical foramen
- Gingival sulcus
- Periodontium:
- Gingiva:
- free or interdental
- marginal
- alveolar
- Periodontal ligament
- Alveolar bone
- Vessels and nerves:
- dental
- periodontal
- alveolar through alveolar canals.
Visuals
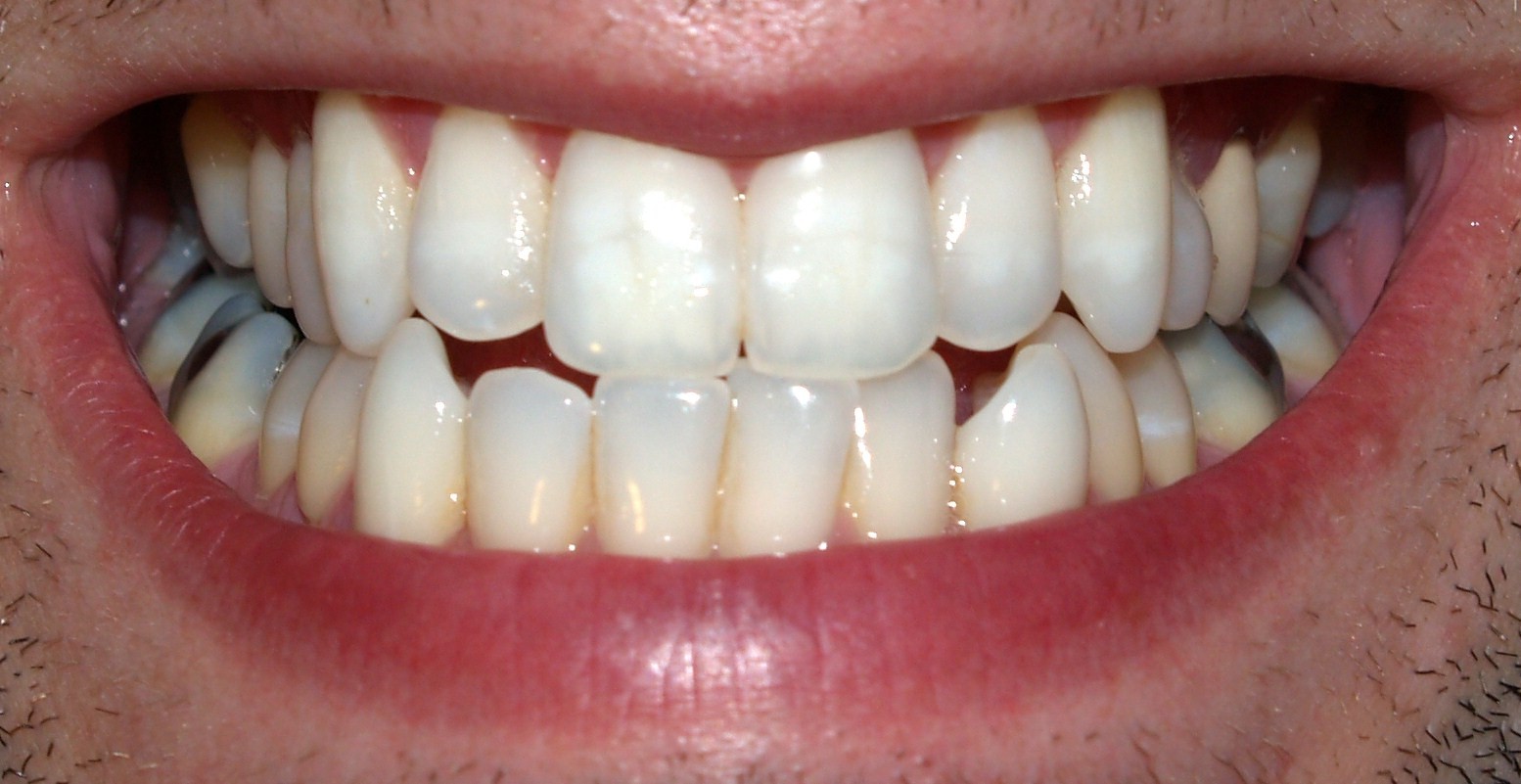
Teeth of humans are small, calcified, hard, whitish structures found in the mouth. They function in [mastication] mechanically breaking down items of food by cutting and crushing them in preparation for swallowing and digestion. The roots of teeth are embedded in the maxilla (upper jaw) or the mandible (lower jaw) and are covered by gingiva or gums. Teeth are made of multiple tissues of varying density and hardness.
Cetaceans
Like human teeth, whale teeth have polyp-like protrusions located on the root surface of the tooth are made of cementum in both animals, but in human teeth, the protrusions are located on the outside of the root, while in whales the nodule is located on the inside of the pulp chamber, with the roots of human teeth made of cementum on the outer surface, whales have cementum on the entire surface of the tooth with a very small layer of enamel at the tip only seen in older whales where the cementum has been worn away to show the underlying enamel.[3]
Mammuthus primigenius
{{free media}}On the right is an image of a molar tooth from a specimen (No. 201669, ELM G319:2) of the woolly mammoth Mammuthus primigenius. The tooth was collected about January 17, 2006, near Puurmani, Estonia, from a Quaternary deposit and the record for the tooth inserted into the collection on October 18, 2008.
The image on the right is of a lophodont cheek molar.
Sahelanthropus tchadensis
"Sahelanthropus tchadensis is one of the oldest known species in the human family tree. This species lived sometime between 7 and 6 million years ago in West-Central Africa (Chad). Walking upright may have helped this species survive in diverse habitats, including forests and grasslands. Although we have only cranial material from Sahelanthropus, studies so far show this species had a combination of ape-like and human-like features. Ape-like features included a small brain (even slightly smaller than a chimpanzee’s), sloping face, very prominent browridges, and elongated skull. Human-like features included small canine teeth, a short middle part of the face, and a spinal cord opening underneath the skull instead of towards the back as seen in non-bipedal apes."[4]
"Some of the oldest evidence of a humanlike species moving about in an upright position comes from Sahelanthropus. The foramen magnum (the large opening where the spinal cord exits out of the cranium from the brain) is located further forward (on the underside of the cranium) than in apes or any other primate except humans. This feature indicates that the head of Sahelanthropus was held on an upright body, probably associated with walking on two legs."[4]
"The first (and, so far, only) fossils of Sahelanthropus are nine cranial specimens from northern Chad. A research team of scientists led by French paleontologist Michael Brunet uncovered the fossils in 2001, including the type specimen TM 266-1-606-1. Before 2001, early humans in Africa had only been found in the Great Rift Valley in East Africa and sites in South Africa, so the discovery of Sahelanthropus fossils in West-Central Africa shows that the earliest humans were more widely distributed than previously thought."[4]
Orrorin tugenensis
"Living around 6 million years ago, Orrorin tugenensis is the one of the oldest early humans on our family tree. Individuals of this species were approximately the size of a chimpanzee and had small teeth with thick enamel, similar to modern humans. The most important fossil of this species is an upper femur, showing evidence of bone buildup typical of a biped - so Orrorin tugenensis individuals climbed trees but also probably walked upright with two legs on the ground."[5]
"A research team led by French paleontologist Brigitte Senut and French geologist Martin Pickford discovered this species in the Tugen Hills region of central Kenya. They found more than a dozen early human fossils dating between about 6.2 million and 6.0 million years old. Because of its novel combination of ape and human traits, the researchers gave a new genus and species name to these fossils, Orrorin tugenensis, which in the local language means “original man in the Tugen region.” So far, Orrorin tugenensis is the only species in the genus Orrorin."[5]
"Orrorin’s femur (thigh bone) and humerus (upper arm bone) are about 1.5 times larger than those of Lucy’s (AL 288-1). Therefore, scientists estimate that Orrorin would have been 1.5 times larger than Au. afarensis, suggesting a size similar to a female chimpanzee, between about 30 and 50 kg."[5]
Horse
An adult horse has between 36 and 44 teeth, where the enamel and dentin layers of horse teeth are intertwined.[6]
All horses have 12 premolars, 12 molars, and 12 incisors.[7]
Generally, all male equines also have four canine teeth (called tushes) between the molars and incisors, but, few female horses (less than 28%) have canines, and those that do usually have only one or two, which many times are only partially erupted.[8]
A few horses have one to four wolf teeth, which are vestigial premolars, with most of those having only one or two, equally common in male and female horses and much more likely to be on the upper jaw; if present these can cause problems as they can interfere with the horse's bit contact; therefore, wolf teeth are commonly removed.[7]
Baboons
"Grossly, human speech concatenates syllables, each with a vowel at its core and each vowel flanked by consonants. Each language has its own particular phonology (i.e. its own inventory of vowel and consonant phonemes and patterns of their use), but the phonemes are drawn systematically from a universal superset structured by the anatomy and physiology of the vocal tract and vocal folds. In particular, all the vowels are differently situated within a roughly triangular [i a u] vocalic space [1,2]."[9]
The procedure for acoustic analysis and VLS labeling is shown in the second image down on the right: "(A) Vocalizations in both human and nonhuman primates use the acoustic signal from the vocal folds vibrating at their fundamental frequency (F0). The formant frequencies depend on the configuration of the vocal tract and the lip opening. (B) [Linear Predictive Coding] LPC analysis was used to reveal the formants of each [vowel like segments] VLS (supplemental information S2 Fig) [28,29]. (C) A Monte Carlo procedure using an n-tube model normalized for the anatomical measures of the baboons’ vocal tracts then served to generate the [Maximal Acoustic Space] MAS (shown by the red line). With this normalized MAS reference, any VLSs could be precisely labeled with the [International Phonetic Alphabet] IPA vowel symbols [30,31]. (D) The VLSs thus labeled correspond to well-documented articulatory configurations with characteristic tongue positions and lip openings. (A-D) Red-&-black dots indicate the corresponding values for this illustrative grunt vocalization, which is classified as [u]."[9]
"[B]aboons’ wahoos, yaks, barks and other vocalizations [contain] evidence of five vowel-like sounds — a sign that the physical capacity for speech may have evolved over much longer timescales than previously thought."[10]
"By comparing the vocal tract of humans and their close primate relatives, researchers can get a sense of which particular traits were necessary for the emergence of speech."[10]
The third image down on the right shows the anatomical "structure of the baboon tongue and muscle recruitment during VLS production: (A) The baboon’s muscle fiber orientation allows tongue motion along two main axes (see also supplemental information S3 Fig). The first axis produces the front/back contrast [æ] ⇔ [u ɔ], including the [u] VLS, which requires a constriction in the back of the vocal tract. Movement along this axis uses antagonistic activation of GGam and SG tongue muscles. The second axis produces the [ɑ] ⇔ [ɨ] VLS contrasts by controlling vertical tongue displacement using the GGp and HG tongue muscles. (B) The baboons’ different VLSs can each be explained by recruitment of a unique configuration of tongue muscles. GGa, GGm, GGp: anterior, medium, posterior part of the genioglossus; HG: hyoglossus; SG: styloglossus."[9]
Speech “engages anatomical traits that might leave fossil clues, as well as overt anatomical, physiological, and behavioral aspects for which parallels can be sought in living primates.” [9]
The fourth image down on the right shows an anatomic sagittal view of the head of a female baboon with vocalization organs labeled: "(1) hyoid bone, (2) air sac, (3) thyroid cartilage, (4) epiglottis, (5) arytenoid cartilage, (6) vocal folds and glottis, (7) cricoid cartilage, (8) trachea, (9) lips, (10) incisors, (11) mandible, (12) hard palate, (13) velum, (14) pharyngeal wall, (15-16-17) anterior GGa, medial GGm, and posterior genioglossus GGp,(18) superior longitudinalis, (19) geniohyoid GH, (20) digastric anterior, (21) C1, (22) C2,(23) C3, (24) mid sagittal line of the vocal tract used to infer the tract length and the computation of the MAS. Note the orientation of the fibers of the GGa, GGm and GGp muscles, which approach vertical on the anterior part of the tongue but are effectively horizontal in the posterior part. The fibers of the styloglossus (SG) muscle on the lateral sides of the tongue have approximately the same inclination as those of a human baby [10]. As in humans, the hyoglossus (HG) muscle has two components which are inserted into the body of the hyoid bone and over the entire extent of the great horn. Its fibers are oriented vertically as found in human children. (N.B.: SG and HG are both lateral to the midline, and do not appear on this view.) This anatomical study shows that a baboon’s tongue has the same musculature as a human’s. Regarding shape and proportions, the baboon’s tongue is more similar to that of a child than that of a human adult."[9]
"In large part, human speech uses vowels as the kernel of a sound and places consonants around those vowels. So the number of different vowels you can make is important, because it means you can make a greater variety of potentially meaningful chunks of sound."[10]
"Think about “cat,” “kit,” “cut,” “coat,” “coot,” “keet,” and “caught” — seven words with distinct meanings. Each has a “k” sound at the beginning and a “t” at the end; what separates them is their vowels. Without each of those subtly distinguishable vowels, English speakers wouldn’t be able to tell those words apart."[10]
"Languages have different inventories and patterns of vowel and consonant usage, but they all rely on roughly the same vocal tract shape. And for a long time, many researchers assumed that nonhuman primates couldn’t make vowel-like sounds because their larynxes (or voice boxes) sat much higher in the neck than human larynxes do. That assumption had major implications for theories on the emergence of language, which remains a uniquely human ability."[10]
“This theory has often been used to buttress the theoretical claim of a recent date for language origin, e.g. 70,000-100,000 years ago. It also diverted scientists' interests away from articulated sound in nonhuman primates as a potential homolog of human speech, and thus lent support to less direct explanations of language evolution, involving communicative gestures, complex cognitive or neural functions, or genetics.”[9]
"Lowered larynxes have been found in other animals that have no ability to make vowel sounds. And human babies, who have very high larynxes, can still generate the same vowel range as adults. Scientists have begun to realize, thanks to computer modeling work, that the movement and control of the tongue’s position is actually much more important in making vowel sounds than the height of the larynx."[10]
Formants "are concentrations of acoustic energy around key frequencies in human speech, and their distribution is defined in part by the shape of our vocal tract."[10]
"The individual formants found in a vowel can tell you the configuration of the mouth that made it — for example, whether the lips are rounded, how high the tongue is, and whether the tongue is pushed forward toward the teeth or back in the mouth."[10]
"In human speech, each vowel has a particular blend of formants that make it a unique, easily identifiable sound."[10]
"15 Guinea baboons (12 females and three males) [such as those in the image on the right, live] in an outdoor enclosure at the National Center for Scientific Research’s primate center in Rousset-sur-Arc, France."[10]
Five "types of baboon vocalizations [appear] to feature formants — grunts, wahoos, barks, yaks and mating calls."[10]
"After analyzing the 1,335 spontaneous vocalizations (and after splitting the wahoos into their wa- and -hoo subunits), the researchers concluded that the recordings held 1,404 “vowel-like segments.”"[10]
"For the ability to make specific vowel-like sounds, it seemed that tongue position really was more important than the larynx’s height."[10]
"The ability to articulate vowel-like sounds, necessary for the development of human speech, was probably shared by the last common ancestor of both humans and baboons [among the Cercopithecoidea] some 25 million years ago."[10]
“Whatever the course of the emergence of language and speech, the evidence developed in this study does not support the hypothesis of the recent, sudden, and simultaneous appearance of language and speech in modern Homo sapiens.”[9]
Dogs
In dogs, the teeth are less likely than humans to form dental caries (cavities) because of the very high pH of dog saliva, which prevents enamel from demineralizing.[11]
Sometimes called cuspids, these teeth are shaped like points (cusps) and are used for tearing and grasping food.[12]
Rodents
Many rodents such as voles and guinea pigs, but not mice, as well as leporidae like rabbits, have continuously growing molars in addition to incisors.[13][14]
Sharks
In cartilaginous fish, such as sharks, the teeth are attached by tough ligaments to the hoops of cartilage] that form the jaw.[2]
Sea urchins
Evolution
Teeth appear to have first evolved in sharks, and are not found in the more primitive jawless fish – while lampreys do have tooth-like structures on the tongue, these are in fact, composed of keratin, not of dentine or enamel, and bear no relationship to true teeth.[2] Though "modern" teeth-like structures with dentine and enamel have been found in late conodonts, they are now supposed to have evolved independently of later vertebrates' teeth.[15][16]
Genes
The genes governing tooth development in mammals are homologous to those involved in the development of fish scales.[17] Study of a tooth plate of a fossil of the extinct fish Romundina stellina showed that the teeth and scales were made of the same tissues, also found in mammal teeth, lending support to the theory that teeth evolved as a modification of scales.[18]
Human genes
GeneID: 765 carbonic anhydrase 6 [ Homo sapiens (human) ].
"We also found that the haplotype (ACA) (rs2274328, rs17032907 and rs11576766) of the carbonic anhydrase VI was associated with a low number of decayed, missing, and filled teeth index with an odds ratio (95% confidence interval) of 0.635 (0.440-0.918)."[19]
"The rs17032907 genetic variant and the haplotype (ACA) of CA VI may be associated with dental caries susceptibility."[19]
GeneID: 3479 IGF1 insulin like growth factor 1 [ Homo sapiens (human) ].
"The protein encoded by this gene is similar to insulin in function and structure and is a member of a family of proteins involved in mediating growth and development. The encoded protein is processed from a precursor, bound by a specific receptor, and secreted. Defects in this gene are a cause of insulin-like growth factor I deficiency. Alternative splicing results in multiple transcript variants encoding different isoforms that may undergo similar processing to generate mature protein."[20]
GeneID: 3480 IGF1R insulin like growth factor 1 receptor [ Homo sapiens (human) ].
"IGF-1 regulates the metabolism of hard dental tissue through binding to the IGF-1 receptor on target cells. Furthermore, IGF-binding-protein-3 promotes the accessibility of IGF-1."[21]
"The teeth [showing ongoing development] showed significantly stronger expression of IGF-1 and IGF-1R. The major sources of all of the proteins investigated immunohistochemically in sections of wisdom teeth were odontoblasts, cementoblasts and cell colonies in the pulpal mesenchyme. [...] members of the IGF-1 family are involved in the late stage of tooth development and the process of pulpal differentiation."[21]
GeneID: 7124 TNF tumor necrosis factor [ Homo sapiens (human) ].
"Tumor necrosis factor-α (TNF-α) is involved in various inflammatory processes, including periodontitis. Although the influences of TNF-α on periodontal ligament fibroblasts and osteoblasts have been widely documented, its effects on cementoblasts, the cells responsible for cementum production, remain largely unknown."[22]
"TNF-α suppressed the mineralization ability of cementoblasts by inhibiting differentiation and inducing apoptosis."[22]
"Various signaling pathways [image on the right], such as p53, PP2AC, p38, Erk1/2, JNK, PI3K-Akt, and NF-κB, were activated during this process. The use of a specific inhibitor and siRNA transfection confirmed that the effects of TNF-α on differentiation and apoptosis in cementoblasts were partially abrogated by inhibiting p53 activity. By contrast, the effects of TNF-α were even exacerbated by the inhibition of the p38, Erk1/2, JNK, PI3K-Akt, and NF-κB pathways. Moreover, p53 activity was further enhanced by blocking the p38, Erk1/2, JNK, and PI3K-Akt signaling pathways."[22]
The "differentiation inhibition and apoptosis in cementoblasts induced by TNF-α were partially dependent on p53 activity. The p38, Erk1/2, JNK, PI3K-Akt, and NF-κB pathways were also activated but acted as balancing players to limit rather than conduct the negative effects of TNF-α. These balancing effects were dependent, or at least partially dependent, on p53, except for the NF-κB pathway."[22]
Prehistory
Teeth are a common fossil that occurs in many strata in the history of Draft:rocks on Draft:Earth.
The prehistory period dates from around 7 x 106 b2k to about 7,000 b2k.
Hypotheses
- The teeth of Tyrannosaurus rex come from the same gene as those of the sea perch, Tautogolabrus adspersus.
See also
- Animal physiology (10 kB) (4 March 2020)
- Anthropology (Aftonian, 540 ka) (30 kB) (6 January 2020)
- Archaeology (-12th Century) (75 kB) (19 January 2020)
- Biology (25 kB) (15 January 2020)
- Cenozoic geochronology (Danian) (231 kB) (23 January 2020)
- Dates (Hadean) (173 kB) (31 August 2019)
- Genes (23 kB) (4 November 2019)
- Human teeth (None) (31 kB) (19 January 2020)
- Libyan history (Oligocene) (30 kB) (1 February 2020)
- Mammalogy (Oligocene) (29 kB) (4 March 2020)
- Middle Ages (-10th century) (108 kB)
- Orthomolecular medicine (17 kB) (17 March 2020)
- Paleanthropology (Paleogene) (195 kB) (27 January 2020)
- Paleontology (Hadean) (119 kB) (21 January 2020)
- Phosphate biochemistry (59 kB) (5 November 2019)
- Phosphate budgets (47 kB) (5 November 2019)
- Phosphate reactions (86 kB) (17 February 2020)
References
- ↑ tooth. San Francisco, California: Wikimedia Foundation, Inc. June 11, 2014. Retrieved 2014-07-01.
- ↑ 2.0 2.1 2.2 Alfred Sherwood Romer and Thomas S. Parsons (1977). The Vertebrate Body. Philadelphia, PA: Holt-Saunders International. pp. 300–310. ISBN 978-0-03-910284-5.
- ↑ Common Characteristics Of Whale Teeth. Retrieved 18 July 2014.
- ↑ 4.0 4.1 4.2 Michael Brunet (26 May 2015). Sahelanthropus tchadensis. Washington, DC USA: Smithsonian Institution. Retrieved 2015-05-31.
- ↑ 5.0 5.1 5.2 Brigitte Senut and Martin Pickford (26 May 2015). Orrorin tugenensis. Washington, DC USA: Smithsonian Institution. Retrieved 2015-05-31.
- ↑ Gummed Out: Young Horses Lose Many Teeth, Vet Says. Retrieved 6 July 2014.
- ↑ 7.0 7.1 Patricia Pence (2002). Equine Dentistry: A Practical Guide. Baltimore: Lippincott Williams & Wilkins. ISBN 978-0-683-30403-9.
- ↑ Al Cirelli (2000). "Equine Dentition" (PDF). Nevada: University of Nevada. Retrieved 7 June 2010.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 Louis-Jean Boë, Frédéric Berthommier, Thierry Legou, Guillaume Captier, Caralyn Kemp, Thomas R. Sawallis, Yannick Becker, Arnaud Rey, Joël Fagot (11 January 2017). "Evidence of a Vocalic Proto-System in the Baboon (Papio papio) Suggests Pre-Hominin Speech Precursors". PLOS One. 12 (1): e0169321. doi:10.1371/journal.pone.0169321. Retrieved 2017-01-19.
- ↑ 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 10.10 10.11 10.12 10.13 Amina Khan (11 January 2017). Vowel sounds made by baboons show that the roots of human speech may go back 25 million years. Los Angeles, California USA: Los Angeles Times. Retrieved 2017-01-19.
- ↑ Hale, FA (2009). "Dental caries in the dog". Can. Vet. J. 50 (12): 1301–4. PMC 2777300. PMID 20190984.
- ↑ Types of Teeth, Dental Anatomy & Tooth Anatomy | Colgate®. www.colgate.com.
- ↑ Tummers M, Thesleff I (March 2003). "Root or crown: a developmental choice orchestrated by the differential regulation of the epithelial stem cell niche in the tooth of two rodent species" (PDF). Development. 130 (6): 1049–57. doi:10.1242/dev.00332. PMID 12571097. Retrieved 28 February 2017.
- ↑ Hunt AM (1959). "A description of the molar teeth and investing tissues of normal guinea pigs". Journal of Dent. Res. 38 (2): 216–31. doi:10.1177/00220345590380020301. PMID 13641521.
- ↑ McCOLLUM, MELANIE; SHARPE, PAUL T. (July 2001). "Evolution and development of teeth". Journal of Anatomy. 199 (1–2): 153–159. doi:10.1046/j.1469-7580.2001.19910153.x. PMC 1594990. PMID 11523817.
- ↑ nature.com, Fossil scans reveal origins of teeth, 16 October 2013
- ↑ Sharpe, P. T. (2001). "Fish scale development: Hair today, teeth and scales yesterday?". Current Biology. 11 (18): R751–R752. doi:10.1016/S0960-9822(01)00438-9. PMID 11566120.
- ↑ Jennifer Viegas (June 24, 2015). First-known teeth belonged to fierce fish. Australian Broadcasting Corporation (ABC) Science. Retrieved June 28, 2015.
- ↑ 19.0 19.1 ZQ Li, XP Hu, JY Zhou, XD Xie, JM Zhang (2015). "Genetic polymorphisms in the carbonic anhydrase VI gene and dental caries susceptibility". Genetics and Molecular Research. 14 (2): 5986–93. doi:10.4238/2015.June.1.16. PMID 26125798. Retrieved 2016-11-27. Unknown parameter
|month=ignored (help) - ↑ RefSeq (September 2015). "IGF1 insulin like growth factor 1 [ Homo sapiens (human) ]". Bethesda, MD, USA: National Center for Biotechnology Information, U.S. National Library of Medicine. Retrieved 2 July 2019.
- ↑ 21.0 21.1 G Magnucki, U Schenk, S Ahrens, A Navarrete Santos, CR Gernhardt, HG Schaller, C Hoang-Vu (2013). "Expression of the IGF-1, IGFBP-3 and IGF-1 receptors in dental pulp stem cells and impacted third molars". Journal of Oral Science. 55 (4): 319–27. PMID 24351920. Retrieved 2015-12-10.
- ↑ 22.0 22.1 22.2 22.3 YL Wang, H He, ZJ Liu, ZG Cao, XY Wang, K Yang, Y Fang, M Han, C Zhang, FY Huo (2015). "Effects of TNF-α on Cementoblast Differentiation, Mineralization, and Apoptosis". Journal of Dental Research. 94 (9): 1225–32. PMID 26088424. Retrieved 2015-12-10. Unknown parameter
|month=ignored (help)
External links
- CS1 maint: Multiple names: authors list
- Pages with citations using unsupported parameters
- Pages with broken file links
- Animals
- Anthropology/Lectures
- Archaeology
- Biochemistry
- Eukaryotes
- Evolution
- Genes
- Humans
- Materials science
- Medical Terminology
- Nursing
- Paleontology
- Phosphates
- Resources last modified in December 2020
- Zoology