Simeprevir
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Simeprevir is a hepatitis C virus NS3/4A protease inhibitor that is FDA approved for the treatment of chronic hepatitis C (CHC) genotype 1 infection as a component of a combination antiviral treatment regimen. Common adverse reactions include pruritus, rash, nausea, hyperbilirubinemia, headache, insomnia, and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Simeprevir is a hepatitis C virus (HCV) NS3/4A protease inhibitor indicated for the treatment of chronic hepatitis C (CHC) genotype 1 infection as a component of a combination antiviral treatment regimen.
Limitations of Use:
- Simeprevir mono therapy is not recommended.
- Simeprevir efficacy in combination with peg interferon alfa and ribavirin (RBV) is substantially reduced in patients infected with HCV genotype 1a with an NS3 Q80K polymorphism at baseline compared to patients infected with hepatitis C virus (HCV) genotype 1a without the Q80K polymorphism. Screening patients with HCV genotype 1a infection for the presence of virus with the NS3 Q80K polymorphism at baseline is strongly recommended. Alternative therapy should be considered for patients infected with HCV genotype 1a containing the Q80K polymorphism.
- Simeprevir is not recommended in patients with severe hepatic impairment (Child-Pugh Class C) due to substantial increases in simeprevir exposures, which have been associated with increased frequency of adverse reactions.
- Simeprevir is not recommended in patients who have previously failed therapy with a treatment regimen that included simeprevir or other HCV protease inhibitors.
Dosage
Simeprevir Combination Treatment
Administer simeprevir in combination with other antiviral drugs for the treatment of CHC infection. For specific dosing recommendations for the antiviral drugs used in combination with simeprevir, refer to their respective prescribing information. Simeprevir monotherapy is not recommended. Administer simeprevir in combination with either:
- Peg-IFN-alfa and RBV: Table 1 displays the recommended dosage regimen and treatment duration of simeprevir in combination with Peg-IFN-alfa and RBV. Refer to Table 3 for treatment stopping rules for simeprevir combination therapy with Peg-IFN-alfa and RBV; or
- Sofosbuvir: Table 2 displays the recommended dosage regimen and treatment duration of simeprevir in combination with sofosbuvir.
The recommended dosage of simeprevir is one capsule taken orally once daily with food. The capsule should be swallowed as a whole.
Testing Prior to Initiation of simeprevir in HCV Genotype 1a-Infected Patients
Prior to initiation of treatment with simeprevir with Peg-IFN-alfa and RBV, screening patients with HCV genotype 1a infection for the presence of virus with the NS3 Q80K polymorphism is strongly recommended and alternative therapy should be considered for patients infected with HCV genotype 1a containing the Q80K polymorphism. Prior to initiation of treatment with simeprevir with sofosbuvir, screening patients infected with HCV genotype 1a for the presence of virus with the NS3 Q80K polymorphism is not strongly recommended but may be considered.
Discontinuation of Dosing
Use with Peg-IFN-Alfa and RBV
During treatment, HCV RNA levels should be monitored as clinically indicated using a sensitive assay with a lower limit of quantification of at least 25 IU/mL.
Because patients with an inadequate on-treatment virologic response (i.e., HCV RNA ≥ 25 IU/mL) are not likely to achieve a sustained virologic response (SVR), discontinuation of treatment is recommended in these patients. Table 3 presents treatment stopping rules for patients who experience an inadequate on-treatment virology response at Weeks 4, 12, and 24.
Dosage Adjustment or Interruption
To prevent treatment failure, avoid reducing the dosage of simeprevir or interrupting treatment. If treatment with simeprevir is discontinued because of adverse reactions or inadequate on-treatment virologic response, simeprevir treatment must not be reinitiated.
If adverse reactions potentially related to the antiviral drug(s) used in combination with simeprevir occur, refer to the instructions outlined in their respective prescribing information for recommendations on dosage adjustment or interruption.
If any of the other antiviral drugs used in combination with simeprevir for the treatment of CHC infection are permanently discontinued for any reason, simeprevir should also be discontinued.
Hepatic Impairment
No dosage recommendation can be made for patients with moderate hepatic impairment (Child-Pugh Class B) due to modest increases in simeprevir exposures. Simeprevir is not recommended for patients with severe hepatic impairment (Child-Pugh Class C) due to substantially higher simeprevir exposures. In clinical trials, higher simeprevir exposures have been associated with increased frequency of adverse reactions, including rash and photosensitivity.
The safety and efficacy of simeprevir have not been studied in HCV-infected patients with moderate or severe hepatic impairment (Child-Pugh Class B or C). Do not administer simeprevir in combination with Peg-IFN-alfa and RBV in patients with decompensated cirrhosis (moderate or severe hepatic impairment). The potential risks and benefits of simeprevir should be carefully considered prior to use in patients with moderate hepatic impairment.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Simeprevir in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Simeprevir in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The safety and efficacy of simeprevir in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of simeprevir in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Simeprevir in pediatric patients.
Contraindications
There are no specific contraindications to simeprevir. However, as simeprevir should always be administered in combination with other antiviral drugs for the treatment of chronic hepatitis C infection, prescribers should consult the complete prescribing information for these drugs for a description of contraindications.
Warnings
Risk of Serious Adverse Reactions Associated With Combination Treatment
Simeprevir should be used in combination with other antiviral drugs for the treatment of CHC infection. Therefore, consult the prescribing information for these drugs before starting therapy with simeprevir. Warnings and Precautions related to these drugs also apply to their use in simeprevir combination treatment.
Photosensitivity
Photosensitivity reactions have been observed with simeprevir combination therapy. Serious photosensitivity reactions resulting in hospitalization have been observed with simeprevir in combination with Peg-IFN-alfa and ribavirin. Photosensitivity reactions occurred most frequently in the first 4 weeks of treatment, but can occur at any time during treatment. Photosensitivity may present as an exaggerated sunburn reaction, usually affecting areas exposed to light (typically the face, "V" area of the neck, extensor surfaces of the forearms, and dorsa of the hands). Manifestations may include burning, erythema, exudation, blistering, and edema.
Use sun protective measures and limit sun exposure during treatment with simeprevir. Avoid use of tanning devices during treatment with simeprevir. Discontinuation of simeprevir should be considered if a photosensitivity reaction occurs and patients should be monitored until the reaction has resolved. If a decision is made to continue simeprevir in the setting of a photosensitivity reaction, expert consultation is advised.
Rash
Rash has been observed with simeprevir combination therapy. Rash occurred most frequently in the first 4 weeks of treatment, but can occur at any time during treatment. Severe rash and rash requiring discontinuation of simeprevir have been reported in subjects receiving simeprevir in combination with Peg-IFN-alfa and RBV. Most of the rash events in Simeprevir-treated patients were of mild or moderate severity. Patients with mild to moderate rashes should be followed for possible progression of rash, including the development of mucosal signs (e.g., oral lesions, conjunctivitis) or systemic symptoms. If the rash becomes severe, simeprevir should be discontinued. Patients should be monitored until the rash has resolved.
Sulfa Allergy
Simeprevir contains a sulfonamide moiety. In subjects with a history of sulfa allergy (n=16), no increased incidence of rash or photosensitivity reactions has been observed. However, there are insufficient data to exclude an association between sulfa allergy and the frequency or severity of adverse reactions observed with the use of simeprevir.
Risk of Adverse Reactions or Reduced Therapeutic Effect Due to Drug Interactions
Co-administration of simeprevir with substances that are moderate or strong inducers or inhibitors of cytochrome P450 3A (CYP3A) is not recommended as this may lead to significantly lower or higher exposure of simeprevir, respectively, which may result in reduced therapeutic effect or adverse reactions
Adverse Reactions
Clinical Trials Experience
Simeprevir should be administered in combination with other antiviral drugs. Refer to the prescribing information of the antiviral drugs used in combination with simeprevir for a description of adverse reactions associated with their use.
The following serious and otherwise important adverse drug reactions (ADRs) are discussed in detail in another section of the labeling:
- Photosensitivity
- Rash
Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
Adverse Reactions when Used in Combination with Peg-IFN-Alfa and RBV
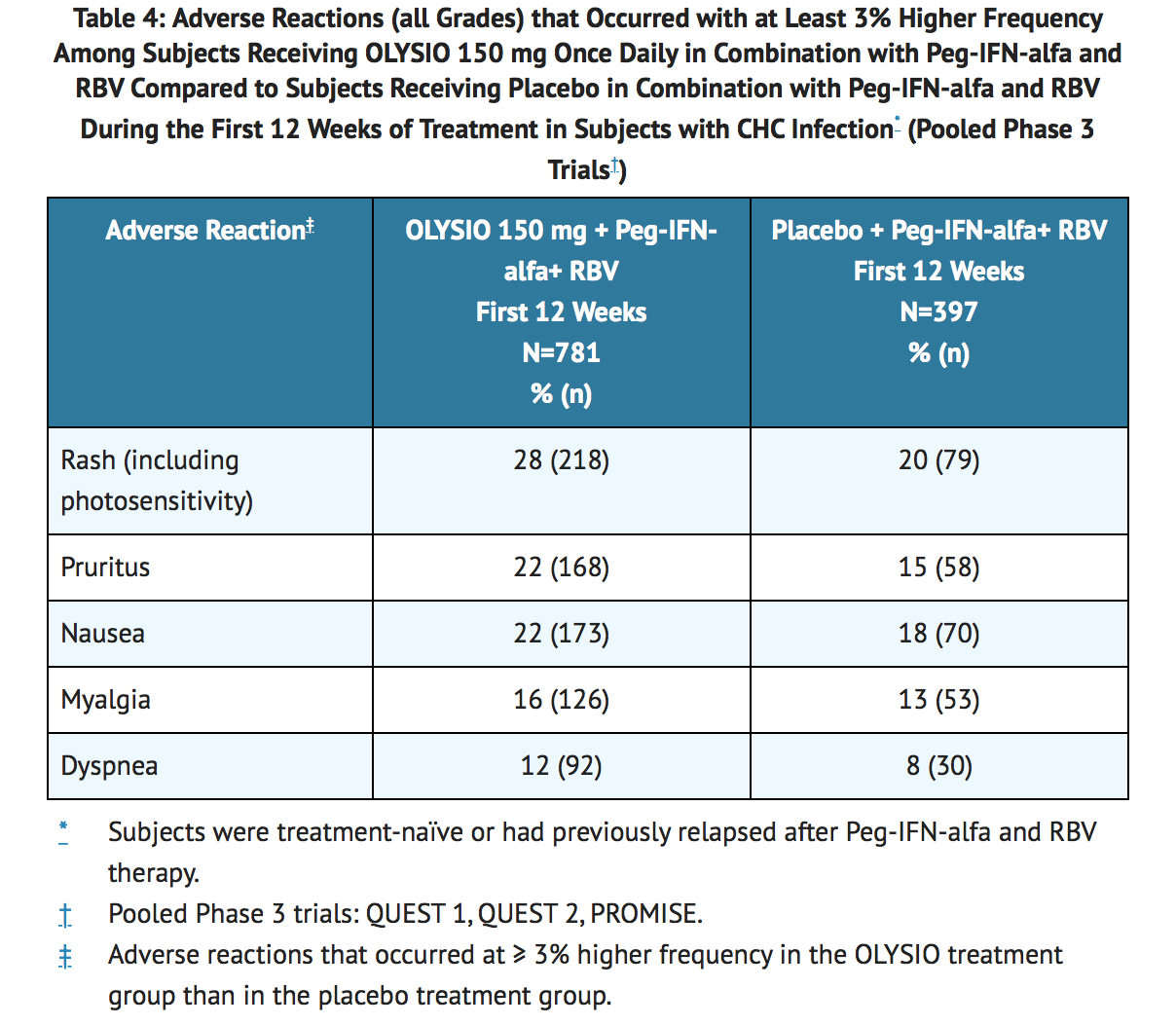
The safety profile of simeprevir in combination with Peg-IFN-alfa and RBV in patients with HCV genotype 1 infection who were treatment-naïve or who had previously relapsed following interferon therapy with or without RBV is based on pooled data from three Phase 3 trials. These trials included a total of 1178 subjects who received simeprevir or placebo in combination with 24 or 48 weeks of Peg-IFN-alfa and RBV. Of the 1178 subjects, 781 subjects were randomized to receive simeprevir 150 mg once daily for 12 weeks and 397 subjects were randomized to receive placebo once daily for 12 weeks.
In the pooled Phase 3 safety data, the majority of the adverse reactions reported during 12 weeks treatment with simeprevir in combination with Peg-IFN-alfa and RBV were Grade 1 to 2 in severity. Grade 3 or 4 adverse reactions were reported in 23% of subjects receiving simeprevir in combination with Peg-IFN-alfa and RBV versus 25% of subjects receiving placebo in combination with Peg-IFN-alfa and RBV. Serious adverse reactions were reported in 2% of subjects receiving simeprevir in combination with Peg-IFN-alfa and RBV and in 3% of subjects receiving placebo in combination with Peg-IFN-alfa and RBV. Discontinuation of simeprevir or placebo due to adverse reactions occurred in 2% and 1% of subjects receiving simeprevir with Peg-IFN-alfa and RBV and subjects receiving placebo with Peg-IFN-alfa and RBV, respectively.
The following table lists adverse reactions (all Grades) that occurred with at least 3% higher frequency among subjects receiving simeprevir 150 mg once daily in combination with Peg-IFN-alfa and RBV, compared to subjects receiving placebo in combination with Peg-IFN-alfa and RBV, during the first 12 weeks of treatment in the pooled Phase 3 trials in subjects who were treatment-naïve or who had previously relapsed after Peg-IFN-alfa and RBV therapy (see TABLE 4).
Rash and Photosensitivity
In the Phase 3 clinical trials, rash (including photosensitivity reactions) was observed in 28% of Simeprevir-treated subjects compared to 20% of placebo-treated subjects during the 12 weeks of treatment with simeprevir or placebo in combination with Peg-IFN-alfa and RBV. Fifty-six percent (56%) of rash events in the simeprevir group occurred in the first 4 weeks, with 42% of cases occurring in the first 2 weeks. Most of the rash events in Simeprevir-treated subjects were of mild or moderate severity (Grade 1 or Grade 2). Severe (Grade 3) rash occurred in 1% of Simeprevir-treated subjects and in none of the placebo-treated subjects. There were no reports of life-threatening (Grade 4) rash. Discontinuation of simeprevir or placebo due to rash occurred in 1% of Simeprevir-treated subjects, compared to less than 1% of placebo-treated subjects. The frequencies of rash and photosensitivity reactions were higher in subjects with higher simeprevir exposures.
All subjects enrolled in the Phase 3 trials were directed to use sun protection measures. In these trials, adverse reactions under the specific category of photosensitivity were reported in 5% of Simeprevir-treated subjects compared to 1% of placebo-treated subjects during the 12 weeks of treatment with simeprevir or placebo in combination with Peg-IFN-alfa and RBV. Most photosensitivity reactions in Simeprevir-treated subjects were of mild or moderate severity (Grade 1 or 2). Two Simeprevir-treated subjects experienced photosensitivity reactions which resulted in hospitalization. No life-threatening photosensitivity reactions were reported.
Dyspnea
During the 12 weeks of treatment with Simeprevir, dyspnea was reported in 12% of Simeprevir-treated subjects compared to 8% of placebo-treated subjects (all grades; pooled Phase 3 trials). All dyspnea events reported in Simeprevir-treated subjects were of mild or moderate severity (Grade 1 or 2). There were no Grade 3 or 4 dyspnea events reported and no subjects discontinued treatment with simeprevir due to dyspnea. Sixty-one percent (61%) of dyspnea events occurred in the first 4 weeks of treatment with simeprevir.
Laboratory abnormalities
There were no differences between treatment groups for the following laboratory parameters: hemoglobin, neutrophils, platelets, aspartate aminotransferase, alanine aminotransferase, amylase, or serum creatinine. Laboratory abnormalities that were observed at a higher incidence in Simeprevir-treated subjects than in placebo-treated subjects are listed in Table 5.
Elevations in bilirubin were predominately mild to moderate (Grade 1 or 2) in severity, and included elevation of both direct and indirect bilirubin. Elevations in bilirubin occurred early after treatment initiation, peaking by study Week 2, and were rapidly reversible upon cessation of simeprevir. Bilirubin elevations were generally not associated with elevations in liver transaminases.
Adverse Reactions when Used with Sofosbuvir
In the COSMOS trial, the most common (> 10%) adverse reactions reported during 12 weeks treatment with simeprevir in combination with sofosbuvir without RBV were fatigue (25%), headache (21%), nausea (21%), insomnia (14%) and pruritus (11%). Rash and photosensitivity were reported in 11% and 7% of subjects, respectively. During 24 weeks treatment with simeprevir in combination with sofosbuvir, dizziness (16%), and diarrhea (16%) were also commonly reported.
Postmarketing Experience
There is limited information regarding Simeprevir Postmarketing Experience in the drug label.
Drug Interactions
Potential for simeprevir to Affect Other Drugs
Simeprevir mildly inhibits CYP1A2 activity and intestinal CYP3A4 activity, but does not affect hepatic CYP3A4 activity. Co-administration of simeprevir with drugs that are primarily metabolized by CYP3A4 may result in increased plasma concentrations of such drugs (see TABLE 6). Simeprevir does not affect CYP2C9, CYP2C19 or CYP2D6 in vivo.
Simeprevir inhibits OATP1B1/3 and P-glycoprotein (P-gp) transporters. Co-administration of simeprevir with drugs that are substrates for OATP1B1/3 and P-gp transport may result in increased plasma concentrations of such drugs (see TABLE 6).
Potential for Other Drugs to Affect Simeprevir
The primary enzyme involved in the biotransformation of simeprevir is CYP3A. Clinically relevant effects of other drugs on simeprevir pharmacokinetics via CYP3A may occur. Co-administration of simeprevir with moderate or strong inhibitors of CYP3A may significantly increase the plasma exposure of simeprevir. Co-administration with moderate or strong inducers of CYP3A may significantly reduce the plasma exposure of simeprevir and lead to loss of efficacy (see TABLE 6). Therefore, co-administration of simeprevir with substances that are moderate or strong inducers or inhibitors of CYP3A is not recommended.
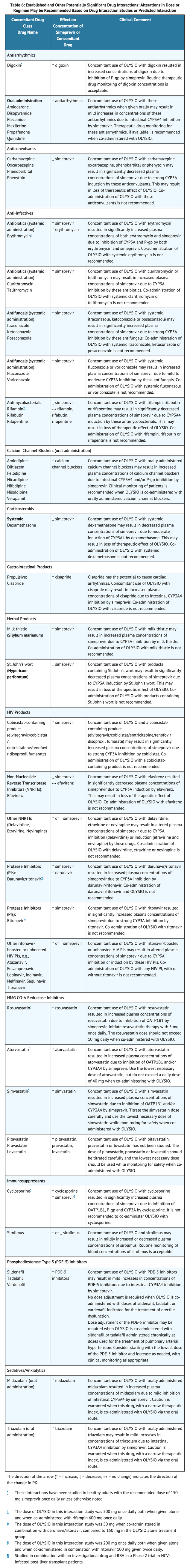
Established and Other Potentially Significant Drug Interactions
Table 6 shows the established and other potentially significant drug interactions based on which alterations in dose or regimen of simeprevir and/or co-administered drug may be recommended. Drugs that are not recommended for co-administration with simeprevir are also included in Table 6. For information regarding the magnitude of interaction, see TABLES 7 and 8.
Drugs without Clinically Significant Interactions with Simeprevir
In addition to the drugs included in Table 6, the interaction between simeprevir and the following drugs were evaluated in clinical studies and no dose adjustments are needed for either drug caffeine, dextromethorphan, escitalopram, ethinyl estradiol/norethindrone, methadone, midazolam (intravenous administration), omeprazole, raltegravir, rilpivirine, sofosbuvir, tacrolimus, tenofovir, disoproxil fumarate, and warfarin.
No clinically relevant drug-drug interaction is expected when simeprevir is co-administered with antacids, the corticosteroids budesonide, fluticasone, methylprednisolone, and prednisone, fluvastatin, H2-receptor antagonists, the narcotic analgesics buprenorphine and naloxone, NRTIs (such as abacavir, didanosine, emtricitabine, lamivudine, stavudine, zidovudine), maraviroc, methylphenidate, and proton pump inhibitors.
Use in Specific Populations
Pregnancy
Pregnancy
Simeprevir must be administered in combination with other antiviral drugs. Refer to prescribing information of the drugs used in combination with simeprevir for information regarding use in pregnancy.
Pregnancy Category C
Risk Summary
Adequate and well-controlled trials with simeprevir have not been conducted in pregnant women. In animal reproduction studies with simeprevir, embryofetal developmental toxicity was observed at drug exposures higher than human exposure at the recommended clinical dose. Simeprevir should be used during pregnancy only if the potential benefit justifies the potential risk. Female patients of childbearing potential should use an effective contraceptive method.
If simeprevir is administered with Peg-IFN-alfa and RBV, refer to the prescribing information for Peg-IFN-alfa and RBV for information on use in pregnancy.
Animal Data
Simeprevir showed no teratogenicity in rats and mice at exposures 0.5 times (in rats) and 6 times (in mice) the mean area under the plasma concentration time curve (AUC) in humans at the recommended dose of 150 mg once daily.
In a mouse embryofetal study at doses up to 1000 mg/kg, simeprevir resulted in early and late in utero fetal losses and early maternal deaths at an exposure approximately 6 times higher than the mean AUC in humans at the recommended 150 mg daily dose. Significantly decreased fetal weights and an increase in fetal skeletal variations were seen at exposures approximately 4 times higher than the mean AUC in humans at the recommended daily dose.
In a rat pre- and postnatal study, maternal animals were exposed to simeprevir during gestation and lactation at doses up to 1000 mg/kg/day. In pregnant rats, simeprevir resulted in early deaths at 1000 mg/kg/day corresponding to exposures similar to the mean AUC in humans at the recommended 150 mg once daily dose. Significant reduction in body weight gain was seen at an exposure 0.7 times the mean AUC in humans at the recommended 150 mg once daily dose. The developing rat offspring exhibited significantly decreased body weight and negative effects on physical growth (delay and small size) and development (decreased motor activity) following simeprevir exposure in utero (via maternal dosing) and during lactation (via maternal milk to nursing pups) at a maternal exposure similar to the mean AUC in humans at the recommended 150 mg once daily dose. Subsequent survival, behavior and reproductive capacity were not affected.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Simeprevir in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Simeprevir during labor and delivery.
Nursing Mothers
Nursing Mothers
It is not known whether simeprevir or its metabolites are present in human breast milk. When administered to lactating rats, simeprevir was detected in plasma of suckling rats likely due to excretion of simeprevir via milk. Because of the potential for adverse reactions from the drug in nursing infants, a decision must be made whether to discontinue nursing or discontinue treatment with Simeprevir, taking into account the importance of the therapy to the mother.
If simeprevir is administered in a regimen containing RBV, the information for RBV with regard to nursing mothers also applies to this combination regimen.
Pediatric Use
The safety and efficacy of simeprevir in pediatric patients have not been established.
Geriatic Use
Clinical studies of simeprevir did not include sufficient numbers of patients older than 65 years to determine whether they respond differently from younger patients. No dose adjustment of simeprevir is required in geriatric patients.
Gender
There is no FDA guidance on the use of Simeprevir with respect to specific gender populations.
Race
Patients of East Asian ancestry exhibit higher simeprevir exposures. In clinical trials, higher simeprevir exposures have been associated with increased frequency of adverse reactions, including rash and photosensitivity. There are insufficient safety data to recommend an appropriate dose for patients of East Asian ancestry. The potential risks and benefits of simeprevir should be carefully considered prior to use in patients of East Asian ancestry.
Renal Impairment
No dose adjustment of simeprevir is required in patients with mild, moderate or severe renal impairment. The safety and efficacy of simeprevir have not been studied in HCV-infected patients with severe renal impairment (creatinine clearance below 30 mL/min) or end-stage renal disease, including patients requiring dialysis. Simeprevir is highly protein-bound; therefore, dialysis is unlikely to result in significant removal of simeprevir.
Refer to the prescribing information for the other antiviral drug(s) used in combination with simeprevir regarding their use in patients with renal impairment.
Hepatic Impairment
No dose adjustment of simeprevir is required in patients with mild hepatic impairment (Child-Pugh Class A). The safety and efficacy of simeprevir have not been studied in HCV-infected patients with moderate or severe hepatic impairment (Child-Pugh Class B or C). No dosage recommendation can be given for patients with moderate hepatic impairment (Child-Pugh Class B) due to modest increases in simeprevir exposures. Simeprevir is not recommended for patients with severe hepatic impairment (Child-Pugh Class C) due to substantially higher simeprevir exposures.
In clinical trials, higher simeprevir exposures have been associated with increased frequency of adverse reactions, including rash and photosensitivity. The potential risks and benefits of simeprevir should be carefully considered prior to use in patients with moderate hepatic impairment.
Refer to the prescribing information for the antiviral drug(s) used in combination with simeprevir regarding their use in patients with hepatic impairment. The combination of simeprevir with Peg-IFN-alfa and RBV is contraindicated in patients with decompensated cirrhosis (moderate or severe hepatic impairment).
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Simeprevir in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Simeprevir in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
- During treatment, HCV RNA levels should be monitored as clinically indicated using a sensitive assay with a lower limit of quantification of at least 25 IU/mL.
Because patients with an inadequate on-treatment virology response (i.e., HCV RNA ≥ 25 IU/mL) are not likely to achieve a sustained virology response (SVR), discontinuation of treatment is recommended in these patients.
- Discontinuation of simeprevir should be considered if a photosensitivity reaction occurs and patients should be monitored until the reaction has resolved. If a decision is made to continue simeprevir in the setting of a photosensitivity reaction, expert consultation is advised.
- Discontinuation of simeprevir should be considered if the rash becomes severePatients should be monitored until the rash has resolved.
- Concomitant use of simeprevir with digoxin resulted in increased concentrations of digoxin due to inhibition of P-gp by simeprevir. Routine therapeutic drug monitoring of digoxin concentrations is acceptable.
- Concomitant use of simeprevir with these antiarrhythmics when given orally may result in mild increases in concentrations of these antiarrhythmics due to intestinal CYP3A4 inhibition by simeprevir. Therapeutic drug monitoring for these antiarrhythmics, if available, is recommended when co-administered with simeprevir.
- Clinical monitoring of patients is recommended when simeprevir is co-administered with orally administered calcium channel blockers.
- Concomitant use of simeprevir with simvastatin resulted in increased plasma concentrations of simvastatin. Titrate the simvastatin dose carefully and use the lowest necessary dose of simvastatin while monitoring for safety when co-administered with simeprevir.
- Concomitant use of simeprevir with pitavastatin, pravastatin or lovastatin has not been studied. The dose of pitavastatin, pravastatin or lovastatin should be titrated carefully and the lowest necessary dose should be used while monitoring for safety when co-administered with simeprevir.
- Concomitant use of simeprevir and sirolimus may result in mildly increased or decreased plasma concentrations of sirolimus. Routine monitoring of blood concentrations of sirolimus is acceptable.
- Dose adjustment of the PDE-5 inhibitor may be required when simeprevir is co-administered with sildenafil or tadalafil administered chronically at doses used for the treatment of pulmonary arterial hypertension. Consider starting with the lowest dose of the PDE-5 inhibitor and increase as needed, with clinical monitoring as appropriate.
IV Compatibility
There is limited information regarding the compatibility of Simeprevir and IV administrations.
Overdosage
Human experience of overdose with simeprevir is limited. There is no specific antidote for overdose with simeprevir. In the event of an overdose, the patient's clinical status should be observed and the usual supportive measures employed.
Simeprevir is highly protein-bound; therefore, dialysis is unlikely to result in significant removal of simeprevir.
Pharmacology
| |
Simeprevir
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | J05 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 749.94 g/mol |
| SMILES | & |
| Synonyms | TMC435; TMC435350 |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
Simeprevir is a direct-acting antiviral (DAA) agent against the hepatitis C virus.
Structure
OLYSIO (simeprevir) is an inhibitor of the HCV NS3/4A protease.
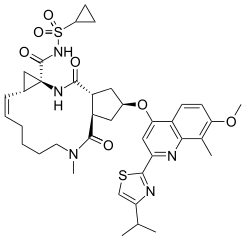
The chemical name for simeprevir is (2R,3aR,10Z,11aS,12aR,14aR)-N-(cyclopropylsulfonyl)-2-2-(4-isopropyl-1,3-thiazol-2-yl)-7-methoxy-8-methyl-4-quinolinyl]oxy]-5-methyl-4,14-dioxo-2,3,3a,4,5,6,7,8,9,11a,12,13,14,14a-tetradecahydrocyclopenta[c]cyclopropa[g][1,6]diazacyclotetradecine-12a(1H)-carboxamide. Its molecular formula is C38H47N5O7S2 and its molecular weight is 749.94. Simeprevir has the following structural formula:

Chemical Structure
Simeprevir drug substance is a white to almost white powder. Simeprevir is practically insoluble in water over a wide pH range. It is practically insoluble in propylene glycol, very slightly soluble in ethanol, and slightly soluble in acetone. It is soluble in dichloromethane and freely soluble in some organic solvents (e.g., tetrahydrofuran and N,N-dimethylformamide).
OLYSIO (simeprevir) for oral administration is available as 150 mg strength hard gelatin capsules. Each capsule contains 154.4 mg of simeprevir sodium salt, which is equivalent to 150 mg of simeprevir. OLYSIO (simeprevir) capsules contain the following inactive ingredients: colloidal anhydrous silica, croscarmellose sodium, lactose monohydrate, magnesium stearate and sodium lauryl sulphate. The white capsule contains gelatin and titanium dioxide (E171) and is printed with ink containing iron oxide black (E172) and shellac (E904).
Pharmacodynamics
Cardiac Electrophysiology
The effect of simeprevir at the recommended dose of 150 mg once daily and 350 mg (at 2.3 times the recommended dosage) once daily for 7 days on the QT interval was evaluated in a randomized, double-blind, placebo- and positive-controlled (moxifloxacin 400 mg once daily), 4-way cross-over study in 60 healthy subjects. No meaningful changes in QTc interval were observed at either the recommended dose of 150 mg once daily or the dose of 350 mg (2.3 times the recommended dosage) once daily.
Pharmacokinetics
The pharmacokinetic properties of simeprevir have been evaluated in healthy adult subjects and in adult HCV-infected subjects. Plasma Cmax and AUC increased more than dose-proportionally after multiple doses between 75 mg and 200 mg once daily, with accumulation occurring following repeated dosing. Steady-state was reached after 7 days of once daily dosing. Plasma exposure (AUC) of simeprevir in HCV-infected subjects was about 2- to 3-fold higher compared to that observed in HCV-uninfected subjects. Plasma Cmax and AUC of simeprevir were similar during co-administration of simeprevir with Peg-IFN-alfa and RBV compared with administration of simeprevir alone. In HCV-infected subjects, the mean steady-state predose plasma concentration was 1936 ng/mL (standard deviation: 2640) and the mean steady-state AUC24 was 57469 ng.h/mL (standard deviation: 63571).
Absorption
Simeprevir is orally bioavailable. Maximum plasma concentrations (Cmax) are typically achieved between 4 to 6 hours post dose.
In vitro studies with human Caco-2 cells indicated that simeprevir is a substrate of P-gp.
Effects of Food on Oral Absorption
Administration of simeprevir with food to healthy subjects increased the relative bioavailability (AUC) by 61% and 69% after a high-fat, high-caloric (928 kcal) and normal-caloric (533 kcal) breakfast, respectively, and delayed the absorption by 1 hour and 1.5 hours, respectively. Due to increased bioavailability, simeprevir should be administered with food. The type of food does not affect exposure to simeprevir.
Distribution
Simeprevir is extensively bound to plasma proteins (greater than 99.9%), primarily to albumin and, to a lesser extent, alfa 1-acid glycoprotein. Plasma protein binding is not meaningfully altered in patients with renal or hepatic impairment.
In animals, simeprevir is extensively distributed to gut and liver (liver:blood ratio of 29:1 in rat) tissues. In vitro data and physiologically-based pharmacokinetic modeling and simulations indicate that hepatic uptake in humans is mediated by OATP1B1/3.
Metabolism
Simeprevir is metabolized in the liver. In vitro experiments with human liver microsomes indicated that simeprevir primarily undergoes oxidative metabolism by the hepatic CYP3A system. Involvement of CYP2C8 and CYP2C19 cannot be excluded. Co-administration of simeprevir with moderate or strong inhibitors of CYP3A may significantly increase the plasma exposure of simeprevir, and co-administration with moderate or strong inducers of CYP3A may significantly reduce the plasma exposure of simeprevir.
Following a single oral administration of 200 mg (1.3 times the recommended dosage) 14C-simeprevir to healthy subjects, the majority of the radioactivity in plasma (mean: 83%) was accounted for by unchanged drug and a small part of the radioactivity in plasma was related to metabolites (none being major metabolites). Metabolites identified in feces were formed via oxidation at the macrocyclic moiety or aromatic moiety or both and by O-demethylation followed by oxidation.
Elimination
Elimination of simeprevir occurs via biliary excretion. Renal clearance plays an insignificant role in its elimination. Following a single oral administration of 200 mg 14C-simeprevir to healthy subjects, on average 91% of the total radioactivity was recovered in feces. Less than 1% of the administered dose was recovered in urine. Unchanged simeprevir in feces accounted for on average 31% of the administered dose.
The terminal elimination half-life of simeprevir was 10 to 13 hours in HCV-uninfected subjects and 41 hours in HCV-infected subjects receiving 200 mg (1.3 times the recommended dosage) of simeprevir.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
Simeprevir was not genotoxic in a series of in vitro and in vivo tests including the Ames test, the mammalian forward mutation assay in mouse lymphoma cells or the in vivo mammalian micronucleus test. Carcinogenicity studies with simeprevir have not been conducted.
If Simeprevir is administered in a combination regimen containing RBV, refer to the prescribing information for RBV for information on carcinogenesis and mutagenesis.
Impairment of Fertility
In a rat fertility study at doses up to 500 mg/kg/day, 3 male rats treated with simeprevir (2/24 rats at 50 mg/kg/day and 1/24 rats at 500 mg/kg/day) showed no motile sperm, small testes and epididymides, and resulted in infertility in 2 out of 3 of the male rats at approximately 0.2 times the mean AUC in humans.
If Simeprevir is administered with Peg-IFN-alfa and RBV, refer to the prescribing information for Peg-IFN-alfa and RBV for information on impairment of fertility.
Animal Toxicology and/or Pharmacology
Cardiovascular toxicity consisting of acute endocardial and myocardial necrosis restricted to the left ventricular subendocardial area was seen in 2 out of 6 animals in a 2-week oral dog toxicity study at an exposure approximately 28 times the mean AUC in humans at the recommended daily dose of 150 mg. No cardiac findings were observed in a 6-month and a 9-month oral toxicity study at exposures, respectively, of 11 and 4 times the mean AUC in humans at the recommended daily dose of 150 mg.
If Simeprevir is administered in a combination regimen containing sofosbuvir, refer to the prescribing information for sofosbuvir for information on animal toxicology.
Clinical Studies
Overview of Clinical Trials
The efficacy of Simeprevir in combination with Peg-IFN-alfa and RBV in patients with HCV genotype 1 infection was evaluated in two Phase 3 trials in treatment-naïve subjects (trials QUEST 1 and QUEST 2), one Phase 3 trial in subjects who relapsed after prior interferon-based therapy (PROMISE) and one Phase 2 trial in subjects who failed prior therapy with Peg-IFN and RBV (including prior relapsers, partial and null responders) (ASPIRE). Prior relapsers were subjects who had HCV RNA not detected at the end of prior IFN-based therapy and HCV RNA detected during follow-up; prior partial responders were subjects with prior on-treatment greater than or equal to 2 log10 reduction in HCV RNA from baseline at Week 12 and HCV RNA detected at the end of prior therapy with Peg-IFN and RBV; and null responders were subjects with prior on-treatment less than 2 log10 reduction in HCV RNA from baseline at Week 12 during prior therapy with Peg-IFN and RBV. Subjects in these trials had compensated liver disease (including cirrhosis), HCV RNA of at least 10000 IU/mL, and liver histopathology consistent with CHC infection. In subjects who were treatment-naïve and prior relapsers, the overall duration of treatment with Peg-IFN-alfa and RBV in the Phase 3 trials was response-guided. In these subjects, the planned total duration of HCV treatment was 24 weeks if the following on-treatment protocol-defined response-guided therapy (RGT) criteria were met: HCV RNA lower than 25 IU/mL (detected or not detected) at Week 4 AND HCV RNA not detected at Week 12. Plasma HCV RNA levels were measured using the Roche COBAS® TaqMan® HCV test (version 2.0), for use with the High Pure System (25 IU/mL lower limit of quantification and 15 IU/mL limit of detection). Treatment stopping rules for HCV therapy were used to ensure that subjects with inadequate on-treatment virologic response discontinued treatment in a timely manner.
The efficacy of Simeprevir in combination with sofosbuvir without or with RBV was evaluated in a Phase 2 trial (COSMOS) in HCV genotype 1 infected prior null responders with METAVIR fibrosis score F0–F4 or treatment-naïve subjects with METAVIR fibrosis score F3–F4 and compensated liver disease.
SVR was defined as HCV RNA not detected 24 weeks after planned end of treatment (SVR24) in the ASPIRE trial and was defined as HCV RNA lower than 25 IU/mL detected or not detected 12 weeks after the planned end of treatment (SVR12) in the COSMOS and Phase 3 trials.
Treatment with Simeprevir in Combination with Peg-IFN-alfa and RBV
Treatment-Naïve Adult Subjects with HCV Genotype 1 Infection
The efficacy of Simeprevir in treatment-naïve patients with HCV genotype 1 infection was demonstrated in two randomized, double-blind, placebo-controlled, 2-arm, multicenter, Phase 3 trials (QUEST 1 and QUEST 2). The designs of both trials were similar. All subjects received 12 weeks of once daily treatment with 150 mg Simeprevir or placebo, plus Peg-IFN-alfa-2a (QUEST 1 and QUEST 2) or Peg-IFN-alfa-2b (QUEST 2) and RBV, followed by 12 or 36 weeks of therapy with Peg-IFN-alfa and RBV in accordance with the on-treatment protocol-defined RGT criteria. Subjects in the control groups received 48 weeks of Peg-IFN-alfa-2a or -2b and RBV.
In the pooled analysis for QUEST 1 and QUEST 2, demographics and baseline characteristics were balanced between both trials and between the Simeprevir and placebo treatment groups. In the pooled analysis of trials (QUEST 1 and QUEST 2), the 785 enrolled subjects had a median age of 47 years (range: 18 to 73 years); 56% were male; 91% were White, 7% Black or African American, 1% Asian, and 17% Hispanic; 23% had a body mass index (BMI) greater than or equal to 30 kg/m2; 78% had HCV RNA levels greater than 800000 IU/mL; 74% had METAVIR fibrosis score F0, F1 or F2, 16% METAVIR fibrosis score F3, and 10% METAVIR fibrosis score F4 (cirrhosis); 48% had HCV genotype 1a, and 51% HCV genotype 1b; 29% had IL28B CC genotype, 56% IL28B CT genotype, and 15% IL28B TT genotype; 17% of the overall population and 34% of the subjects with genotype 1a virus had the NS3 Q80K polymorphism at baseline. In QUEST 1, all subjects received Peg-IFN-alfa-2a; in QUEST 2, 69% of the subjects received Peg-IFN-alfa-2a and 31% received Peg-IFN-alfa-2b.
Table 11 shows the response rates in treatment-naïve adult subjects with HCV genotype 1 infection. In the Simeprevir treatment group, SVR12 rates were lower in subjects with genotype 1a virus with the NS3 Q80K polymorphism at baseline compared to subjects infected with genotype 1a virus without the Q80K polymorphism.

In the pooled analysis of QUEST 1 and QUEST 2, 88% (459/521) of Simeprevir-treated subjects were eligible for a total treatment duration of 24 weeks. In these subjects, the SVR12 rate was 88% (405/459).
Seventy-eight percent (78%; 404/521) of Simeprevir-treated subjects had HCV RNA not detected at Week 4 (RVR); in these subjects the SVR12 rate was 90% (362/404), while 8% (32/392) with HCV RNA not detected at end of treatment had viral relapse.
SVR12 rates were higher for the Simeprevir treatment group compared to the placebo treatment group by sex, age, race, BMI, HCV genotype/subtype, baseline HCV RNA load (less than or equal to 800000 IU/mL, greater than 800000 IU/mL), METAVIR fibrosis score, and IL28B genotype. Table 12 shows the SVR rates by METAVIR fibrosis score.

SVR12 rates were higher for subjects receiving Simeprevir with Peg-IFN-alfa-2a or Peg-IFN-alfa-2b and RBV (88% and 78%, respectively) compared to subjects receiving placebo with Peg-IFN-alfa-2a or Peg-IFN-alfa-2b and RBV (62% and 42%, respectively) (QUEST 2).
Adult Subjects with HCV Genotype 1 Infection who Failed Prior Peg-IFN-alfa and RBV Therapy
The PROMISE trial was a randomized, double-blind, placebo-controlled, 2-arm, multicenter, Phase 3 trial in subjects with HCV genotype 1 infection who relapsed after prior IFN-based therapy. All subjects received 12 weeks of once daily treatment with 150 mg Simeprevir or placebo, plus Peg-IFN-alfa-2a and RBV, followed by 12 or 36 weeks of therapy with Peg-IFN-alfa-2a and RBV in accordance with the protocol-defined RGT criteria. Subjects in the control group received 48 weeks of Peg-IFN-alfa-2a and RBV.
Demographics and baseline characteristics were balanced between the Simeprevir and placebo treatment groups. The 393 subjects enrolled in the PROMISE trial had a median age of 52 years (range: 20 to 71 years); 66% were male; 94% were White, 3% Black or African American, 2% Asian, and 7% Hispanic; 26% had a BMI greater than or equal to 30 kg/m2; 84% had HCV RNA levels greater than 800000 IU/mL; 69% had METAVIR fibrosis score F0, F1 or F2, 15% METAVIR fibrosis score F3, and 15% METAVIR fibrosis score F4 (cirrhosis); 42% had HCV genotype 1a, and 58% HCV genotype 1b; 24% had IL28B CC genotype, 64% IL28B CT genotype, and 12% IL28B TT genotype; 13% of the overall population and 31% of the subjects with genotype 1a virus had the NS3 Q80K polymorphism at baseline. The prior IFN-based HCV therapy was Peg-IFN-alfa-2a/RBV (68%) or Peg-IFN-alfa-2b/RBV (27%).
Table 13 shows the response rates for the Simeprevir and placebo treatment groups in adult subjects with HCV genotype 1 infection who relapsed after prior interferon-based therapy. In the Simeprevir treatment group, SVR12 rates were lower in subjects infected with genotype 1a virus with the NS3 Q80K polymorphism at baseline compared to subjects infected with genotype 1a virus without the Q80K polymorphism.

In PROMISE, 93% (241/260) of Simeprevir-treated subjects were eligible for a total treatment duration of 24 weeks. In these subjects, the SVR12 rate was 83% (200/241).
Seventy-seven percent (77%; 200/260) of Simeprevir-treated subjects had HCV RNA not detected at Week 4 (RVR); in these subjects the SVR12 rate was 87% (173/200), while 13% (25/196) with HCV RNA not detected at end of treatment had viral relapse.
SVR12 rates were higher for the Simeprevir treatment group compared to the placebo treatment group by sex, age, race, BMI, HCV genotype/subtype, baseline HCV RNA load (less than or equal to 800000 IU/mL, greater than 800000 IU/mL), prior HCV therapy, METAVIR fibrosis score, and IL28B genotype. Table 14 shows the SVR rates by METAVIR fibrosis score.

The ASPIRE trial was a randomized, double-blind, placebo-controlled, 7-arm, Phase 2 trial in subjects with HCV genotype 1 infection, who failed prior therapy with Peg-IFN-alfa and RBV (including prior relapsers, partial responders or null responders). Subjects received 12, 24 or 48 weeks of 100 mg or 150 mg Simeprevir in combination with 48 weeks of Peg-IFN-alfa-2a and RBV, or 48 weeks of placebo in combination with 48 weeks of Peg-IFN-alfa-2a and RBV.
Demographics and baseline characteristics were balanced between the Simeprevir and placebo treatment groups. The 462 subjects enrolled in the ASPIRE trial had a median age of 50 years (range: 20 to 69 years); 67% were male; 93% were White, 5% Black or African American, and 2% Asian; 25% had a BMI greater than or equal to 30 kg/m2; 86% had HCV RNA levels greater than 800000 IU/mL; 63% had METAVIR fibrosis score F0, F1, or F2, 19% METAVIR fibrosis score F3, and 18% METAVIR fibrosis score F4 (cirrhosis); 41% had HCV genotype 1a, and 58% HCV genotype 1b; 18% had IL28B CC genotype, 65% IL28B CT genotype, and 18% IL28B TT genotype (information available for 328 subjects); 12% of the overall population and 27% of the subjects with genotype 1a virus had the NS3 Q80K polymorphism at baseline. Forty percent (40%) of subjects were prior relapsers, 35% prior partial responders, and 25% prior null responders following prior therapy with Peg-IFN-alfa and RBV. One hundred ninety-nine subjects received Simeprevir 150 mg once daily (pooled analysis) of which 66 subjects received Simeprevir for 12 weeks and 66 subjects received placebo in combination with Peg-IFN-alfa and RBV.
Table 15 shows the response rates for the OLYSIO and placebo treatment groups in prior relapsers, prior partial responders and prior null responders.

In prior partial responders, SVR24 rates in subjects receiving Simeprevir with Peg-IFN-alfa and RBV were 47% and 77% in subjects with HCV genotype 1a and 1b, respectively, compared to 13% and 7%, respectively, in subjects receiving placebo with Peg-IFN-alfa and RBV. In prior null responders, SVR24 rates in subjects receiving Simeprevir with Peg-IFN-alfa and RBV were 41% and 47% in subjects with HCV genotype 1a and 1b, respectively, compared to 0% and 33%, respectively, in subjects receiving placebo with Peg-IFN-alfa and RBV.
SVR24 rates were higher in the Simeprevir-treated subjects compared to subjects receiving placebo in combination with Peg-IFN-alfa and RBV, regardless of HCV geno/subtype, METAVIR fibrosis score, and IL28B genotype.
Simeprevir in Combination with Sofosbuvir
Adult Subjects with HCV Genotype 1 Infection
The COSMOS trial was an open-label, randomized Phase 2 trial to investigate the efficacy and safety of 12 or 24 weeks of Simeprevir (150 mg once daily) in combination with sofosbuvir (400 mg once daily) without or with RBV in HCV genotype 1-infected prior null responders with METAVIR fibrosis score F0–F2 (Cohort 1), or treatment-naïve subjects and prior null responders with METAVIR fibrosis score F3–F4 and compensated liver disease (Cohort 2).
The 80 enrolled subjects without advanced hepatic fibrosis in Cohort 1 had a median age of 56 years (range 27 to 70 years; with 8% above 65 years); 61% were male; 71% were White, 29% Black or African American, and 25% were Hispanic; 30% had a BMI greater than or equal to 30 kg/m2; 98% had HCV RNA levels greater than 800,000 IU/mL; 41% had METAVIR fibrosis score F0 or F1 and 59% had METAVIR fibrosis score F2; 78% had HCV genotype 1a, and the remaining patients had HCV genotype 1b; 39% of the overall population and 50% of the subjects with genotype 1a had the NS3 Q80K polymorphism at baseline; 6% had IL28B CC genotype, 70% IL28B CT genotype, and 24% IL28B TT genotype. All subjects were prior null responders to Peg-IFN-alfa and RBV.
The 87 enrolled subjects with advanced hepatic fibrosis in Cohort 2 had a median age of 58 years (range 28 to 70 years; with 3% above 65 years); 67% were male; 91% were White, 9% Black or African American, and 17% were Hispanic; 44% had a BMI greater than or equal to 30 kg/m2; 84% had HCV RNA levels greater than 800,000 IU/mL; 53% had METAVIR fibrosis score F3 and 47% had METAVIR fibrosis score F4 (cirrhosis); 78% had HCV genotype 1a, and 22% HCV genotype 1b; 31% of the overall population and 40% of the subjects with genotype 1a had the NS3 Q80K polymorphism at baseline; 21% had IL28B CC genotype, 56% IL28B CT genotype, and 23% IL28B TT genotype. Fifty-four percent of subjects were prior null responders to Peg-IFN-alfa and RBV and 46% were treatment-naïve.
Table 16 shows the response rates by combining prior null responders in Cohort 1 and treatment-naïve subjects and prior null responders in Cohort 2. When treatment arms with and without ribavirin were combined, the overall SVR12 rate was 95% (61/64) in subjects with METAVIR fibrosis score F0-F3 who received 12 weeks treatment of Simeprevir in combination with sofosbuvir with/without RBV when pooling both cohorts. The overall SVR12 rate was 96% (22/23) in subjects with METAVIR fibrosis score F4 who received 24 weeks treatment of Simeprevir in combination with sofosbuvir with/without RBV when pooling both cohorts. Addition of RBV did not increase response rates in comparison with Simeprevir in combination with sofosbuvir alone; and therefore these data are not shown in Table 16.

How Supplied
OLYSIO 150 mg capsules are white, marked with "TMC435 150" in black ink. The capsules are packaged into a bottle containing 28 capsules (NDC 59676-225-28) or a bottle of 7 capsules (emergency supply; NDC 59676-225-07).
Storage
Store simeprevir capsules in the original bottle in order to protect from light at room temperature below 30°C (86°F).
Images
Drug Images
{{#ask: Page Name::Simeprevir |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Simeprevir |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
OLYSIO (oh li see oh) (simeprevir) Capsules
Read this Patient Information before you start taking simeprevir and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
Simeprevir is used in combination with other antiviral medicines for treating chronic hepatitis C infection. When taking simeprevir in combination with peginterferon alfa and ribavirin you should also read those Medication Guides. When taking simeprevir in combination with sofosbuvir, you should also read its Patient Information leaflet.
What is the most important information I should know about Simeprevir?
If you are pregnant, or plan to become pregnant, talk with your healthcare provider before taking simeprevir. It is not known if simeprevir will harm your unborn baby. Also read the Medication Guides for peginterferon alfa and ribavirin if your healthcare provider prescribes these medications for you in combination with simeprevir. Females must use an effective form of birth control during treatment with simeprevir. Talk with your healthcare provider about birth control methods that you may use during treatment with simeprevir. Simeprevir combination treatment may cause rashes and skin reactions to sunlight. These rashes and skin reactions to sunlight can be severe and you may need to be treated in a hospital. Rashes and skin reactions to sunlight are most common during the first 4 weeks of treatment, but can happen at any time during combination treatment with simeprevir.
Use sunscreen, and wear a hat, sunglasses, and protective clothing when you will be exposed to sunlight during treatment with simeprevir. Limit sunlight exposure during treatment with simeprevir. Avoid use of tanning beds, sunlamps, or other types of light therapy during treatment with simeprevir. Call your healthcare provider right away if you get any of the following symptoms: burning, redness, swelling or blisters on your skin mouth sores or ulcers red or inflamed eyes, like "pink eye" (conjunctivitis) You should not take simeprevir alone. Simeprevir should be used together with other medicines to treat chronic hepatitis C infection.
What is OLYSIO?
OLYSIO is a prescription medicine used with other antiviral medicines to treat chronic (lasting a long time) hepatitis C infection in adults. OLYSIO should not be taken alone. It is not known if simeprevir is safe and effective in children under 18 years of age.
Who should not take OLYSIO?
"WHAT IS THE MOST IMPORTANT INFORMATION I SHOULD KNOW ABOUT OLYSIO?"
What should I tell my healthcare provider before taking OLYSIO?
Before taking OLYSIO, tell your healthcare provider if you:
have liver problems other than hepatitis C virus infection have ever taken any medicine to treat hepatitis C virus infection had a liver transplant are receiving phototherapy have any other medical condition are of East Asian descent are breastfeeding. It is not known if simeprevir passes into your breast milk. You and your healthcare provider should decide if you will take simeprevir or breastfeed. You should not do both. Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
OLYSIO and other medicines may affect each other. This can cause you to have too much or not enough simeprevir or other medicines in your body, which may affect the way simeprevir or your other medicines work, or may cause side effects. Do not start taking a new medicine without telling your healthcare provider or pharmacist.
Especially tell your healthcare provider if you take any of the following medicines:
amiodarone (Cordarone, Pacerone), when taken by mouth amlodipine (Norvasc), when taken by mouth atazanavir (Reyataz) atorvastatin (Lipitor, Caduet) carbamazepine (Carbatrol, Epitol, Equetro, Tegretol) cisapride (Propulsid, Propulsid Quicksolv) clarithromycin (Biaxin, Prevpac) cobicistat-containing medicine (Stribild) cyclosporine (Gengraf, Neoral, Sandimmune) darunavir (Prezista) delavirdine mesylate (Rescriptor) dexamethasone, when taken by mouth or given by injection digoxin (Lanoxin) diltiazem (Cardizem, Dilacor XR, Tiazac), when taken by mouth disopyramide (Norpace), when taken by mouth efavirenz (Sustiva, Atripla) erythromycin (E.E.S., Eryc, Ery-Tab, Erythrocin, Erythrocin Stearate), when taken by mouth or when given by injection etravirine (Intelence) felodipine (Plendil), when taken by mouth flecainide (Tambocor), when taken by mouth fluconazole (Diflucan), when taken by mouth or when given by injection fosamprenavir (Lexiva) indinavir (Crixivan) itraconazole (Sporanox, Onmel), when taken by mouth ketoconazole (Nizoral), when taken by mouth lopinavir (Kaletra) lovastatin (Advicor, Altoprev, Mevacor) mexiletine (Mexitil), when taken by mouth midazolam, when taken by mouth milk thistle (Silybum marianum) or products containing milk thistle nelfinavir (Viracept) nevirapine (Viramune, Viramune XR) nicardipine (Cardene), when taken by mouth nifedipine (Adalat CC, Afeditab CR, Procardia), when taken by mouth nisoldipine (Sular), when taken by mouth oxcarbazepine (Oxtellar XR™, Trileptal) phenobarbital (Luminal) phenytoin (Dilantin, Phenytek) pitavastatin (Livalo) posaconazole (Noxafil), when taken by mouth pravastatin (Pravachol) propafenone (Rythmol SR), when taken by mouth quinidine (Nuedexta, Duraquin, Quinaglute), when taken by mouth rifabutin (Mycobutin) rifampin (Rifadin, Rifamate, Rifater, Rimactane) rifapentine (Priftin) ritonavir (Norvir) rosuvastatin (Crestor) saquinavir mesylate (Invirase) sildenafil (Revatio, Viagra) simvastatin (Zocor, Vytorin, Simcor) sirolimus (Rapamune) St. John's wort (Hypericum perforatum) or products containing St. John's wort tadalafil (Adcirca, Cialis) telithromycin (Ketek) tipranavir (Aptivus) triazolam (Halcion), when taken by mouth verapamil (Calan, Covera-HS, Isoptin, Tarka), when taken by mouth voriconazole (Vfend), when taken by mouth or when given by injection This is not a complete list of medicines that could interact with simeprevir. Ask your healthcare provider or pharmacist if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I take OLYSIO?
Take simeprevir exactly as your healthcare provider tells you to take it. Do not change your dose unless your healthcare provider tells you to. Do not stop taking simeprevir unless your healthcare provider tells you to. If you think there is a reason to stop taking OLYSIO, talk to your healthcare provider before doing so. Take 1 simeprevir capsule each day with food. Swallow simeprevir capsules whole. If you miss a dose of simeprevir and it is more than 12 hours until your next dose, take the missed dose as soon as possible with food. Take the next dose of simeprevir at your regular time. If you miss a dose of simeprevir and it is less than 12 hours until your next dose, skip the missed dose. Take the next dose of simeprevir at your regular time. Do not take two doses of simeprevir at the same time to make up for a missed dose. If you take too much OLYSIO, call your healthcare provider right away or go to the nearest hospital emergency room.
What are the possible side effects of OLYSIO?
See "WHAT IS THE MOST IMPORTANT INFORMATION I SHOULD KNOW ABOUT OLYSIO?" The most common side effects of simeprevir when used in combination with peginterferon alfa and ribavirin include:
skin rash. See "WHAT IS THE MOST IMPORTANT INFORMATION I SHOULD KNOW ABOUT OLYSIO?" section of this leaflet. itching nausea The most common side effects of simeprevir when used in combination with sofosbuvir include:
tiredness headache nausea Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all of the possible side effects of simeprevir. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store OLYSIO?
Store simeprevir at room temperature below 86°F (30°C). Store simeprevir in the original bottle to protect it from light. Keep simeprevir and all medicines out of the reach of children.
General information about the safe and effective use of OLYSIO
It is not known if treatment with simeprevir will prevent you from infecting another person with the hepatitis C virus during your treatment. Talk with your healthcare provider about ways to prevent spreading the hepatitis C virus.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use simeprevir for a condition for which it was not prescribed. Do not give your simeprevir to other people, even if they have the same symptoms that you have. It may harm them.
If you would like more information about OLYSIO, talk with your pharmacist or healthcare provider. You can ask your pharmacist or healthcare provider for information about simeprevir that is written for health professionals.
For more information about OLYSIO, go to www.simeprevir.com or call 1-800-526-7736.
What are the ingredients in OLYSIO?
Active ingredient: simeprevir
Inactive ingredients: colloidal anhydrous silica, croscarmellose sodium, lactose monohydrate, magnesium stearate, sodium lauryl sulphate. The white capsule contains gelatin and titanium dioxide (E171) and is printed with ink containing iron oxide black (E172) and shellac (E904).
This Patient Information has been approved by the U.S. Food and Drug Administration.
Precautions with Alcohol
Alcohol-Simeprevir interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
OLYSIO,Galexos, Galexos, and Sovriad
Look-Alike Drug Names
There is limited information about the look alike drug names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.