Methamphetamine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning
See full prescribing information for complete Boxed Warning.
|
Overview
Methamphetamine is a CNS Stimulant that is FDA approved for the treatment of attention deficit disorder with hyperactivity and exogenous obesity. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hypertension, increased heart rate.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Exogenous Obesity
- As a short-term (i.e., a few weeks) adjunct in a regimen of weight reduction based on caloric restriction, for patients in whom obesity is refractory to alternative therapy, e.g., repeated diets, group programs, and other drugs.
Dosing Information
- One 5 mg tablet should be taken one-half hour before each meal. Treatment should not exceed a few weeks in duration. Methamphetamine is not recommended for use as an anorectic agent in children under 12 years of age.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Methamphetamine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Methamphetamine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Attention Deficit Disorder with Hyperactivity
- Methamphetamine hydrochloride tablets are indicated as an integral part of a total treatment program which typically includes other remedial measures (psychological, educational, social) for a stabilizing effect in children over 6 years of age with a behavioral syndrome characterized by the following group of developmentally inappropriate symptoms: moderate to severe distractibility, short attention span, hyperactivity, emotional lability, and impulsivity. The diagnosis of this syndrome should not be made with finality when these symptoms are only of comparatively recent origin. Nonlocalizing (soft) neurological signs, learning disability, and abnormal EEG may or may not be present, and a diagnosis of central nervous system dysfunction may or may not be warranted.
- The limited usefulness of methamphetamine hydrochloride tablets should be weighed against possible risks inherent in use of the drug, such as those described below.
Dosing information
- Methamphetamine hydrochloride tablets are given orally.
- Methamphetamine should be administered at the lowest effective dosage, and dosage should be individually adjusted. Late evening medication should be avoided because of the resulting insomnia.
- For treatment of children 6 years or older with a behavioral syndrome characterized by moderate to severe distractibility, short attention span, hyperactivity, emotional lability and impulsivity: an initial dose of 5 mg methamphetamine hydrochloride tablets once or twice a day is recommended. Daily dosage may be raised in increments of 5 mg at weekly intervals until an optimum clinical response is achieved. The usual effective dose is 20 to 25 mg daily. The total daily dose may be given in two divided doses daily.
- Where possible, drug administration should be interrupted occasionally to determine if there is a recurrence of behavioral symptoms sufficient to require continued therapy.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Methamphetamine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Methamphetamine in pediatric patients.
Contraindications
- Methamphetamine hydrochloride tablets are contraindicated during or within 14 days following the administration of monoamine oxidase inhibitors; hypertensive crisis may result. It is also contraindicated in patients with glaucoma, advanced arteriosclerosis, symptomatic cardiovascular disease, moderate to severe hypertension, hyperthyroidism or known hypersensitivity or idiosyncrasy to sympathomimetic amines. Methamphetamine should not be given to patients who are in an agitated state or who have a history of drug abuse.
Warnings
|
Warning
See full prescribing information for complete Boxed Warning.
|
- Tolerance to the anorectic effect usually develops within a few weeks. When this occurs, the recommended dose should not be exceeded in an attempt to increase the effect; rather, the drug should be discontinued.
Serious Cardiovascular Events
Sudden Death and Preexisting Structural Cardiac Abnormalities or Other Serious Heart Problems
- Children and Adolescents: Sudden death has been reported in association with CNS stimulant treatment at usual doses in children and adolescents with structural cardiac abnormalities or other serious heart problems. Although some serious heart problems alone carry an increased risk of sudden death, stimulant products generally should not be used in children or adolescents with known serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, or other serious cardiac problems that may place them at increased vulnerability to the sympathomimetic effects of a stimulant drug
- Adults: Sudden deaths, stroke, and myocardial infarction have been reported in adults taking stimulant drugs at usual doses for ADHD. Although the role of stimulants in these adult cases is also unknown, adults have a greater likelihood than children of having serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, coronary artery disease, or other serious cardiac problems. Adults with such abnormalities should also generally not be treated with stimulant drugs.
Hypertension and other Cardiovascular Conditions
- Stimulant medications cause a modest increase in average blood pressure (about 2 to 4 mmHg) and average heart rate (about 3 to 6 bpm), and individuals may have larger increases. While the mean changes alone would not be expected to have short-term consequences, all patients should be monitored for larger changes in heart rate and blood pressure. Caution is indicated in treating patients whose underlying medical conditions might be compromised by increases in blood pressure or heart rate, e.g., those with pre-existing hypertension, heart failure, recent myocardial infarction, or ventricular arrhythmia.
Assessing Cardiovascular Status in Patients being Treated with Stimulant Medications
- Children, adolescents, or adults who are being considered for treatment with stimulant medications should have a careful history (including assessment for a family history of sudden death or ventricular arrhythmia) and physical exam to assess for the presence of cardiac disease, and should receive further cardiac evaluation if findings suggest such disease (e.g., electrocardiogram and echocardiogram). Patients who develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease during stimulant treatment should undergo a prompt cardiac evaluation.
Psychiatric Adverse Events
Preexisting Psychosis
- Administration of stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with a preexisting psychotic disorder.
Bipolar Illness
- Particular care should be taken in using stimulants to treat ADHD in patients with comorbid bipolar disorder because of concern for possible induction of a mixed/manic episode in such patients. Prior to initiating treatment with a stimulant, patients with comorbid depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression.
Emergence of New Psychotic or Manic Symptoms
- Treatment emergent psychotic or manic symptoms, e.g., hallucinations, delusional thinking, or mania in children and adolescents without a prior history of psychotic illness or mania can be caused by stimulants at usual doses. If such symptoms occur, consideration should be given to a possible causal role of the stimulant, and discontinuation of treatment may be appropriate. In a pooled analysis of multiple short-term, placebo-controlled studies, such symptoms occurred in about 0.1% (four patients with events out of 3,482 exposed to methylphenidate or amphetamine for several weeks at usual doses) of stimulant-treated patients compared to 0 in placebo-treated patients.
Aggression
- Aggressive behavior or hostility is often observed in children and adolescents with ADHD, and has been reported in clinical trials and the postmarketing experience of some medications indicated for the treatment of ADHD. Although there is no systematic evidence that stimulants cause aggressive behavior or hostility, patients beginning treatment for ADHD should be monitored for the appearance of or worsening of aggressive behavior or hostility.
Long-Term Suppression of Growth
- Careful follow-up of weight and height in children ages 7 to 10 years who were randomized to either methylphenidate or non-medication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and non-medication treated children over 36 months (to the ages of 10 to 13 years), suggests that consistently medicated children (i.e., treatment for 7 days per week throughout the year) have a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this period of development. Published data are inadequate to determine whether chronic use of amphetamines may cause a similar suppression of growth, however, it is anticipated that they likely have this effect as well. Therefore, growth should be monitored during treatment with stimulants, and patients who are not growing or gaining height or weight as expected may need to have their treatment interrupted.
Seizures
- There is some clinical evidence that stimulants may lower the convulsive threshold in patients with prior history of seizures, in patients with prior EEG abnormalities in absence of seizures, and, very rarely, in patients without a history of seizures and no prior EEG evidence of seizures. In the presence of seizures, the drug should be discontinued.
Peripheral Vasculopathy, including Raynaud's phenomenon
- Stimulants, including methamphetamine hydrochloride tablets, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud's phenomenon. Signs and symptoms are usually intermittent and mild; however, very rare sequelae include digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud's phenomenon, were observed in post-marketing reports at different times and at therapeutic doses in all age groups throughout the course of treatment. Signs and symptoms generally improve after reduction in dose or discontinuation of drug. Careful observation for digital changes is necessary during treatment with ADHD stimulants. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
Visual Disturbance
- Difficulties with accommodation and blurring of vision have been reported with stimulant treatment.
Precautions
General
- Methamphetamine hydrochloride tablets should be used with caution in patients with even mild hypertension.
- Methamphetamine should not be used to combat fatigue or to replace rest in normal persons.
- Prescribing and dispensing of methamphetamine should be limited to the smallest amount that is feasible at one time in order to minimize the possibility of overdosage.
Adverse Reactions
Clinical Trials Experience
- The following are adverse reactions in decreasing order of severity within each category that have been reported:
- Cardiovascular: Elevation of blood pressure, tachycardia and palpitation. Fatal cardiorespiratory arrest has been reported, mostly in the context of abuse/misuse.
- Central Nervous System: Psychotic episodes have been rarely reported at recommended doses. Dizziness, dysphoria, overstimulation, euphoria, insomnia, tremor, restlessness and headache. Exacerbation of motor and phonic tics and Tourette's syndrome.
- Gastrointestinal: Diarrhea, constipation, dryness of mouth, unpleasant taste and other gastrointestinal disturbances.
- Miscellaneous: Suppression of growth has been reported with the long-term use of stimulants in children
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Methamphetamine in the drug label.
Drug Interactions
- Insulin requirements in diabetes mellitus may be altered in association with the use of methamphetamine and the concomitant dietary regimen.
- Methamphetamine may decrease the hypotensive effect of guanethidine.
- Methamphetamine hydrochloride tablets should not be used concurrently with monoamine oxidase inhibitors.
- Concurrent administration of tricyclic antidepressants and indirect-acting sympathomimetic amines such as the amphetamines, should be closely supervised and dosage carefully adjusted.
- Phenothiazines are reported in the literature to antagonize the CNS stimulant action of the amphetamines.
Drug/Laboratory Test Interactions
- Literature reports suggest that amphetamines may be associated with significant elevation of plasma corticosteroids. This should be considered if determination of plasma corticosteroid levels is desired in a person receiving amphetamines.
Use in Specific Populations
Pregnancy
Teratogenic effects
- Methamphetamine has been shown to have teratogenic and embryocidal effects in mammals given high multiples of the human dose. There are no adequate and well controlled studies in pregnant women. Methamphetamine hydrochloride tablets should not be used during pregnancy unless the potential benefit justifies the potential risk to the fetus.
Nonteratogenic effects
- Infants born to mothers dependent on amphetamines have an increased risk of premature delivery and low birth weight. Also, these infants may experience symptoms of withdrawal as demonstrated by dysphoria, including agitation and significant lassitude.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Methamphetamine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Methamphetamine during labor and delivery.
Nursing Mothers
- Amphetamines are excreted in human milk. Mothers taking amphetamines should be advised to refrain from nursing.
Pediatric Use
- Safety and effectiveness for use as an anorectic agent in children below the age of 12 years have not been established. Long-term effects of methamphetamine in children have not been established .
- Drug treatment is not indicated in all cases of the behavioral syndrome characterized by moderate to severe distractibility, short attention span, hyperactivity, emotional lability and impulsivity. It should be considered only in light of the complete history and evaluation of the child. The decision to prescribe methamphetamine hydrochloride tablets should depend on the physician's assessment of the chronicity and severity of the child's symptoms and their appropriateness for his/her age. Prescription should not depend solely on the presence of one or more of the behavioral characteristics.When these symptoms are associated with acute stress reactions, treatment with methamphetamine hydrochloride tablets is usually not indicated.
Clinical experience suggests that in psychotic children, administration of methamphetamine hydrochloride tablets may exacerbate symptoms of behavior disturbance and thought disorder.
- Amphetamines have been reported to exacerbate motor and phonic tics and Tourette's syndrome. Therefore, clinical evaluation for tics and Tourette's syndrome in children and their families should precede use of stimulant medications.
Geriatic Use
- Clinical Studies of methamphetamine hydrochloride tablets did not include sufficient numbers of subjects age 65 years and over to determine whether elderly subjects respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy observed in this population.
Gender
There is no FDA guidance on the use of Methamphetamine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Methamphetamine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Methamphetamine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Methamphetamine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Methamphetamine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Methamphetamine in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Methamphetamine in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Methamphetamine in the drug label.
Overdosage
- Manifestations of acute overdosage with methamphetamine include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, panic states, hyperpyrexia, and rhabdomyolysis. Fatigue and depression usually follow the central stimulation. Cardiovascular effects include arrhythmias, hypertension or hypotension, and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea, and abdominal cramps. Fatal poisoning usually terminates in convulsions and coma.
- Consult with a Certified Poison Control Center regarding treatment for up to date guidance and advice. Management of acute methamphetamine intoxication is largely symptomatic and includes gastric evacuation,administration of activated charcoal, and sedation. Experience with hemodialysis or peritoneal dialysis is inadequate to permit recommendations in this regard.
- Acidification of urine increases methamphetamine excretion, but is believed to increase risk of acute renal failure if myoglobinuria is present. Intravenous phentolamine (Regitine1) has been suggested for possible acute, severe hypertension, if this complicates methamphetamine overdosage. Usually a gradual drop in blood pressure will result when sufficient sedation has been achieved. Chlorpromazine has been reported to be useful in decreasing CNS stimulation and sympathomimetic effects.
There is limited information regarding Chronic Overdose of Methamphetamine in the drug label.
Pharmacology
Mechanism of Action
- Methamphetamine is a sympathomimetic amine with CNS stimulant activity. Peripheral actions include elevation of systolic and diastolic blood pressures and weak bronchodilator and respiratory stimulant action. Drugs of this class used in obesity are commonly known as "anorectics" or "anorexigenics". It has not been established, however, that the action of such drugs in treating obesity is primarily one of appetite suppression. Other central nervous system actions, or metabolic effects, may be involved, for example.
Adult obese subjects instructed in dietary management and treated with "anorectic" drugs, lose more weight on the average than those treated with placebo and diet, as determined in relatively short-term clinical trials.
- The magnitude of increased weight loss of drug-treated patients over placebo-treated patients is only a fraction of a pound a week. The rate of weight loss is greatest in the first weeks of therapy for both drug and placebo subjects and tends to decrease in succeeding weeks. The origins of the increased weight loss due to the various possible drug effects are not established. The amount of weight loss associated with the use of an "anorectic" drug varies from trial to trial, and the increased weight loss appears to be related in part to variables other than the drug prescribed, such as the physician-investigator, the population treated, and the diet prescribed. Studies do not permit conclusions as to the relative importance of the drug and non-drug factors on weight loss.
- The natural history of obesity is measured in years, whereas the studies cited are restricted to a few weeks duration; thus, the total impact of drug-induced weight loss over that of diet alone must be considered clinically limited.
- The mechanism of action involved in producing the beneficial behavioral changes seen in hyperkinetic children receiving methamphetamine is unknown.
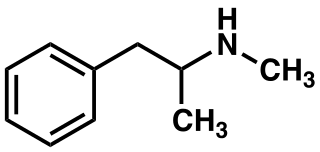
Structure
- Methamphetamine hydrochloride tablets, USP chemically known as (S)-N, α -dimethylbenzeneethanamine hydrochloride, is a member of the amphetamine group of sympathomimetic amines. It has the following structural formula:

- Methamphetamine hydrochloride tablets contain 5 mg of methamphetamine hydrochloride, USP for oral administration.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Methamphetamine in the drug label.
Pharmacokinetics
- In humans, methamphetamine is rapidly absorbed from the gastrointestinal tract. The primary site of metabolism is in the liver by aromatic hydroxylation, N-dealkylation and deamination. At least seven metabolites have been identified in the urine. The biological half-life has been reported in the range of 4 to 5 hours. Excretion occurs primarily in the urine and is dependent on urine pH. Alkaline urine will significantly increase the drug half-life. Approximately 62% of an oral dose is eliminated in the urine within the first 24 hours with about one-third as intact drug and the remainder as metabolites.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Data are not available on long-term potential for carcinogenicity, mutagenicity, or impairment of fertility.
Clinical Studies
There is limited information regarding Clinical Studies of Methamphetamine in the drug label.
How Supplied
- Methamphetamine Hydrochloride Tablets, USP are available containing 5 mg of methamphetamine hydrochloride, USP.
- The 5 mg tablets are white, round, unscored tablets debossed with 115 on one side of the tablet and blank on the other side. They are available as follows:
- NDC 0378-8115-01
bottles of 100 tablets
- Store at 20º to 25ºC (68º to 77ºF). [See USP Controlled Room Temperature.]
- Protect from light.
- Dispense in a tight, light resistant container as defined in the USP using child-resistant closure.
Storage
There is limited information regarding Methamphetamine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Methamphetamine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Methamphetamine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- The patient should be informed that methamphetamine may impair the ability to engage in potentially hazardous activities, such as, operating machinery or driving a motor vehicle.
- Circulation problems in fingers and toes [Peripheral vasculopathy, including Raynaud's phenomenon]
- Instruct patients beginning treatment with methamphetamine hydrochloride tablets about the risk of peripheral vasculopathy, including Raynaud's phenomenon, and associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red.
- Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes.
- Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking methamphetamine hydrochloride tablets.
- Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
- The patient should be cautioned not to increase dosage, except on advice of the physician.
- Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with methamphetamine and should counsel them in its appropriate use. A patient Medication Guide is available for methamphetamine hydrochloride tablets. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have.
Precautions with Alcohol
- Alcohol-Methamphetamine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Desoxyn
Look-Alike Drug Names
There is limited information regarding Methamphetamine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "Properties: Predicted – EP|Suite". Methmphetamine. Chemspider. Retrieved 3 January 2013.
- ↑ "Chemical and Physical Properties". Methamphetamine. PubChem Compound. National Center for Biotechnology Information. Retrieved 31 December 2013.
- ↑ 3.0 3.1 "Toxicity". Methamphetamine. PubChem Compound. National Center for Biotechnology Information. Retrieved 31 December 2013.
- ↑ "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. December 2013. pp. 12–13. Retrieved 30 December 2013.
- ↑ Lemke TL, Williams DA, Roche VF, Zito W (2013). Foye's Principles of Medicinal Chemistry (7th ed. ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 648. ISBN 1609133455.
Alternatively, direct oxidation of amphetamine by DA β-hydroxylase can afford norephedrine.
- ↑ Krueger SK, Williams DE (June 2005). "Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism". Pharmacol. Ther. 106 (3): 357–387. doi:10.1016/j.pharmthera.2005.01.001. PMC 1828602. PMID 15922018.
- ↑
- ↑
- ↑
{{#subobject:
|Page Name=Methamphetamine
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Methamphetamine |Label Name=Methamphetamine04.png
}}
{{#subobject:
|Label Page=Methamphetamine |Label Name=Methamphetamine05.png
}}