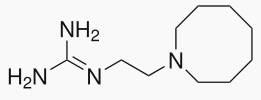
Guanethidine
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a600027 |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 1.5 days |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C10H22N4 |
| Molar mass | 198.309 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Guanethidine |
|
Articles |
|---|
|
Most recent articles on Guanethidine Most cited articles on Guanethidine |
|
Media |
|
Powerpoint slides on Guanethidine |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Guanethidine at Clinical Trials.gov Clinical Trials on Guanethidine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Guanethidine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Guanethidine Discussion groups on Guanethidine Patient Handouts on Guanethidine Directions to Hospitals Treating Guanethidine Risk calculators and risk factors for Guanethidine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Guanethidine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Guanethidine is an antihypertensive drug that reduces the release of catecholamines, such as norepinephrine. Guanethidine is transported across the sympathetic nerve membrane by the same mechanism that transports norepinephrine itself (NET, uptake 1), and uptake is essential for the drug's action. Once guanethidine has entered the nerve, it is concentrated in transmitter vesicles, where it replaces norepinephrine.It may also inhibit the release of granules by decreasing norepinephrine.
Side effects
Side effects include orthostatic and exercise hypotension, sexual dysfunction (delayed or retrograde ejaculation), and diarrhea.
Pharmacology
Guanethidine is transported by uptake 1 into the presynaptic terminal transported by Norepinephrine transporter (NET). (In this it competes with norepinephrine so can potentiate exogenously applied norepinephrine.) It becomes concentrated in norepinephrine transmitter vesicles, replacing norepinephrine in these vesicles. This leads to a gradual depletion of norepinephrine stores in the nerve endings. Once inside the terminal it blocks the release of norepinephrine in response to arrival of an action potential. Spontaneous release is not affected.
Uses
Guanethidine was once a mainstay for hypertension resistant to other agents, and was often used safely during pregnancy, but it is no longer used in the US due to lack of availability. It is still licensed in some countries, e.g., UK, for the rapid control of blood pressure in a hypertensive emergency.
Intravenous nerve block (Bier block) using guanethidine has been used to treat chronic pain caused by complex regional pain syndrome.[1]
Chemical synthesis
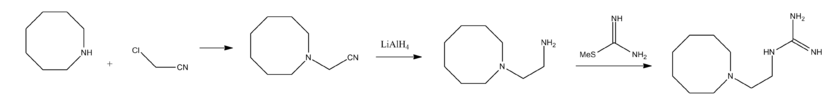
Guanethidine is synthesized beginning with the alkylation of azocine with chloroacetonitrile. This reaction which forms 1-azocinylacetonitrile, which can be reduced into 1-(2-aminoethyl)azocine by using lithium aluminium hydride as a reductant. This compound reacts with S-methylthiourea forming guanethidine.[2][3][4]
References
- ↑ Joyce PI, Rizzi D, Caló G, Rowbotham DJ, Lambert DG (November 2002). "The effect of guanethidine and local anesthetics on the electrically stimulated mouse vas deferens". Anesth. Analg. 95 (5): 1339–43, table of contents. doi:10.1097/00000539-200211000-00045. PMID 12401623.
- ↑ R.P. Mull, Template:US Patent (1960)
- ↑ R.P. Mull, Template:US Patent (1961)
- ↑ R.P. Mull, Template:US Patent (1962)
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Template:drugs.com link with non-standard subpage
- Articles with changed KEGG identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Drug
- Alpha-adrenergic agonists
- Guanidines
- Azocanes