Indinavir warnings and precautions
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mohamed Moubarak, M.D. [2]
Warnings and Precautions
Warnings
ALERT: Find out about medicines that should NOT be taken with CRIXIVAN. This statement is included on the product's bottle label.
- Nephrolithiasis/Urolithiasis
Nephrolithiasis/urolithiasis has occurred with CRIXIVAN therapy. The cumulative frequency of nephrolithiasis is substantially higher in pediatric patients (29%) than in adult patients (12.4%; range across individual trials: 4.7% to 34.4%). The cumulative frequency of nephrolithiasis events increases with increasing exposure to CRIXIVAN; however, the risk over time remains relatively constant. In some cases, nephrolithiasis/urolithiasis has been associated with renal insufficiency or acute renal failure, pyelonephritis with or without bacteremia. If signs or symptoms of nephrolithiasis/urolithiasis occur, (including flank pain, with or without hematuria or microscopic hematuria), temporary interruption (e.g., 1-3 days) or discontinuation of therapy may be considered. Adequate hydration is recommended in all patients treated with CRIXIVAN. (See ADVERSE REACTIONS and DOSAGE AND ADMINISTRATION, Nephrolithiasis/Urolithiasis.)
- Hemolytic Anemia
Acute hemolytic anemia, including cases resulting in death, has been reported in patients treated with CRIXIVAN. Once a diagnosis is apparent, appropriate measures for the treatment of hemolytic anemia should be instituted, including discontinuation of CRIXIVAN.
- Hepatitis
Hepatitis including cases resulting in hepatic failure and death has been reported in patients treated with CRIXIVAN. Because the majority of these patients had confounding medical conditions and/or were receiving concomitant therapy(ies), a causal relationship between CRIXIVAN and these events has not been established.
- Hyperglycemia
New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus and hyperglycemia have been reported during post-marketing surveillance in HIV-infected patients receiving protease inhibitor therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those patients who discontinued protease inhibitor therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and a causal relationship between protease inhibitor therapy and these events has not been established.
- Drug Interactions
Concomitant use of CRIXIVAN with lovastatin or simvastatin is contraindicated due to an increased risk of myopathy including rhabdomyolysis. Caution should be exercised if CRIXIVAN is used concurrently with atorvastatin or rosuvastatin. Titrate the atorvastatin and rosuvastatin doses carefully and use the lowest necessary dose with CRIXIVAN. (See PRECAUTIONS, Drug Interactions.)
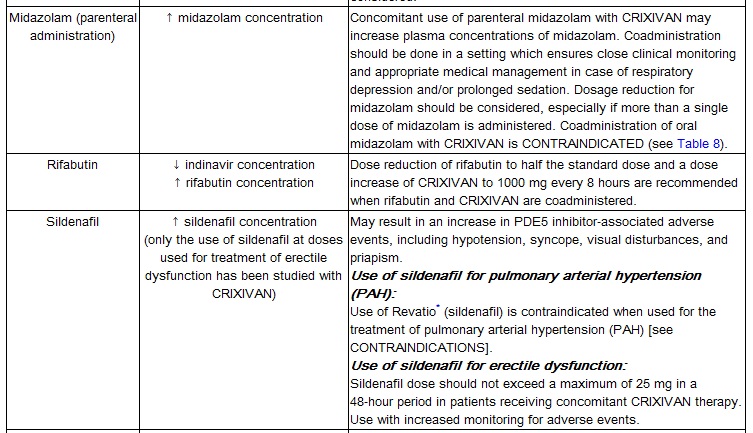
Midazolam is extensively metabolized by CYP3A4. Co-administration with CRIXIVAN with or without ritonavir may cause a large increase in the concentration of this benzodiazepine. No drug interaction study has been performed for the co-administration of CRIXIVAN with benzodiazepines. Based on data from other CYP3A4 inhibitors, plasma concentrations of midazolam are expected to be significantly higher when midazolam is given orally. Therefore CRIXIVAN should not be co-administered with orally administered midazolam (see CONTRAINDICATIONS), whereas caution should be used with co-administration of CRIXIVAN and parenteral midazolam. Data from concomitant use of parenteral midazolam with other protease inhibitors suggest a possible 3-4 fold increase in midazolam plasma levels. If CRIXIVAN with or without ritonavir is co-administered with parenteral midazolam, it should be done in a setting which ensures close clinical monitoring and appropriate medical management in case of respiratory depression and/or prolonged sedation. Dosage reduction for midazolam should be considered, especially if more than a single dose of midazolam is administered.
Particular caution should be used when prescribing sildenafil, tadalafil, or vardenafil in patients receiving indinavir. Coadministration of CRIXIVAN with these medications is expected to substantially increase plasma concentrations of sildenafil, tadalafil, and vardenafil and may result in an increase in adverse events, including hypotension, visual changes, and priapism, which have been associated with sildenafil, tadalafil, and vardenafil (see CONTRAINDICATIONS and PRECAUTIONS, Drug Interactions and Information for Patients, and the manufacturer's complete prescribing information for sildenafil, tadalafil, or vardenafil).
Concomitant use of CRIXIVAN and St. John's wort (Hypericum perforatum) or products containing St. John's wort is not recommended. Coadministration of CRIXIVAN and St. John's wort has been shown to substantially decrease indinavir concentrations (see CLINICAL PHARMACOLOGY, Drug Interactions) and may lead to loss of virologic response and possible resistance to CRIXIVAN or to the class of protease inhibitors.
Precautions
- General
Indirect hyperbilirubinemia has occurred frequently during treatment with CRIXIVAN and has infrequently been associated with increases in serum transaminases (see also ADVERSE REACTIONS, Clinical Trials and Post-Marketing Experience). It is not known whether CRIXIVAN will exacerbate the physiologic hyperbilirubinemia seen in neonates. (See Pregnancy.)
- Tubulointerstitial Nephritis
Reports of tubulointerstitial nephritis with medullary calcification and cortical atrophy have been observed in patients with asymptomatic severe leukocyturia (>100 cells/ high power field). Patients with asymptomatic severe leukocyturia should be followed closely and monitored frequently with urinalyses. Further diagnostic evaluation may be warranted, and discontinuation of CRIXIVAN should be considered in all patients with severe leukocyturia.
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including CRIXIVAN. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of treatment.
- Coexisting Conditions
Patients with hemophilia: There have been reports of spontaneous bleeding in patients with hemophilia A and B treated with protease inhibitors. In some patients, additional factor VIII was required. In many of the reported cases, treatment with protease inhibitors was continued or restarted. A causal relationship between protease inhibitor therapy and these episodes has not been established. (See ADVERSE REACTIONS, Post-Marketing Experience.)
Patients with hepatic insufficiency due to cirrhosis: In these patients, the dosage of CRIXIVAN should be lowered because of decreased metabolism of CRIXIVAN (see DOSAGE AND ADMINISTRATION).
Patients with renal insufficiency: Patients with renal insufficiency have not been studied.
- Fat Redistribution
Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
- Information for Patients
A statement to patients and health care providers is included on the product's bottle label. ALERT: Find out about medicines that should NOT be taken with CRIXIVAN. A Patient Package Insert (PPI) for CRIXIVAN is available for patient information.
CRIXIVAN is not a cure for HIV-1 infection and patients may continue to experience illnesses associated with HIV-1 infection, including opportunistic infections. Patients should remain under the care of a physician when using CRIXIVAN.
Patients should be advised to avoid doing things that can spread HIV-1 infection to others.
- Do not share needles or other injection equipment.
- Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades.
- Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood.
- Do not breastfeed. We do not know if CRIXIVAN can be passed to your baby in your breast milk and whether it could harm your baby. Also, mothers with HIV-1 should not breastfeed because HIV-1 can be passed to the baby in the breast milk.
Patients should be advised to remain under the care of a physician when using CRIXIVAN and should not modify or discontinue treatment without first consulting the physician. Therefore, if a dose is missed, patients should take the next dose at the regularly scheduled time and should not double this dose. Therapy with CRIXIVAN should be initiated and maintained at the recommended dosage.
CRIXIVAN may interact with some drugs; therefore, patients should be advised to report to their doctor the use of any other prescription, non-prescription medication or herbal products, particularly St. John's wort.
For optimal absorption, CRIXIVAN should be administered without food but with water 1 hour before or 2 hours after a meal. Alternatively, CRIXIVAN may be administered with other liquids such as skim milk, juice, coffee, or tea, or with a light meal, e.g., dry toast with jelly, juice, and coffee with skim milk and sugar; or corn flakes, skim milk and sugar (see CLINICAL PHARMACOLOGY, Effect of Food on Oral Absorption and DOSAGE AND ADMINISTRATION). Ingestion of CRIXIVAN with a meal high in calories, fat, and protein reduces the absorption of indinavir.
Patients receiving a phosphodiesterase type 5 (PDE5) inhibitor (sildenafil, tadalafil, or vardenafil) should be advised that they may be at an increased risk of PDE5 inhibitor-associated adverse events including hypotension, visual changes, and priapism, and should promptly report any symptoms to their doctors (see CONTRAINDICATIONS and WARNINGS, Drug Interactions).
Patients should be informed that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy and that the cause and long-term health effects of these conditions are not known at this time.
CRIXIVAN Capsules are sensitive to moisture. Patients should be informed that CRIXIVAN should be stored and used in the original container and the desiccant should remain in the bottle.
- Drug Interactions
Indinavir is an inhibitor of the cytochrome P450 isoform CYP3A4. Coadministration of CRIXIVAN and drugs primarily metabolized by CYP3A4 may result in increased plasma concentrations of the other drug, which could increase or prolong its therapeutic and adverse effects (see CONTRAINDICATIONS and WARNINGS).
Indinavir is metabolized by CYP3A4. Drugs that induce CYP3A4 activity would be expected to increase the clearance of indinavir, resulting in lowered plasma concentrations of indinavir. Coadministration of CRIXIVAN and other drugs that inhibit CYP3A4 may decrease the clearance of indinavir and may result in increased plasma concentrations of indinavir.[1]
- Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies were conducted in mice and rats. In mice, no increased incidence of any tumor type was observed. The highest dose tested in rats was 640 mg/kg/day; at this dose a statistically significant increased incidence of thyroid adenomas was seen only in male rats. At that dose, daily systemic exposure in rats was approximately 1.3 times higher than daily systemic exposure in humans. No evidence of mutagenicity or genotoxicity was observed in in vitro microbial mutagenesis (Ames) tests, in vitro alkaline elution assays for DNA breakage, in vitro and in vivo chromosomal aberration studies, and in vitro mammalian cell mutagenesis assays. No treatment-related effects on mating, fertility, or embryo survival were seen in female rats and no treatment-related effects on mating performance were seen in male rats at doses providing systemic exposure comparable to or slightly higher than that with the clinical dose. In addition, no treatment-related effects were observed in fecundity or fertility of untreated females mated to treated males.
- Pregnancy
Pregnancy Category C:
Developmental toxicity studies were performed in rabbits (at doses up to 240 mg/kg/day), dogs (at doses up to 80 mg/kg/day), and rats (at doses up to 640 mg/kg/day). The highest doses in these studies produced systemic exposures in these species comparable to or slightly greater than human exposure. No treatment-related external, visceral, or skeletal changes were observed in rabbits or dogs. No treatment-related external or visceral changes were observed in rats. Treatment-related increases over controls in the incidence of supernumerary ribs (at exposures at or below those in humans) and of cervical ribs (at exposures comparable to or slightly greater than those in humans) were seen in rats. In all three species, no treatment-related effects on embryonic/fetal survival or fetal weights were observed.
In rabbits, at a maternal dose of 240 mg/kg/day, no drug was detected in fetal plasma 1 hour after dosing. Fetal plasma drug levels 2 hours after dosing were approximately 3% of maternal plasma drug levels. In dogs, at a maternal dose of 80 mg/kg/day, fetal plasma drug levels were approximately 50% of maternal plasma drug levels both 1 and 2 hours after dosing. In rats, at maternal doses of 40 and 640 mg/kg/day, fetal plasma drug levels were approximately 10 to 15% and 10 to 20% of maternal plasma drug levels 1 and 2 hours after dosing, respectively.
Indinavir was administered to Rhesus monkeys during the third trimester of pregnancy (at doses up to 160 mg/kg twice daily) and to neonatal Rhesus monkeys (at doses up to 160 mg/kg twice daily). When administered to neonates, indinavir caused an exacerbation of the transient physiologic hyperbilirubinemia seen in this species after birth; serum bilirubin values were approximately fourfold above controls at 160 mg/kg twice daily. A similar exacerbation did not occur in neonates after in utero exposure to indinavir during the third trimester of pregnancy. In Rhesus monkeys, fetal plasma drug levels were approximately 1 to 2% of maternal plasma drug levels approximately 1 hour after maternal dosing at 40, 80, or 160 mg/kg twice daily.
Hyperbilirubinemia has occurred during treatment with CRIXIVAN (see PRECAUTIONS and ADVERSE REACTIONS). It is unknown whether CRIXIVAN administered to the mother in the perinatal period will exacerbate physiologic hyperbilirubinemia in neonates.
There are no adequate and well-controlled studies in pregnant patients. CRIXIVAN should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
A CRIXIVAN dose of 800 mg every 8 hours (with zidovudine 200 mg every 8 hours and lamivudine 150 mg twice a day) has been studied in 16 HIV-infected pregnant patients at 14 to 28 weeks of gestation at enrollment (study PACTG 358). Given the substantially lower antepartum exposures observed and the limited data in this patient population, indinavir use is not recommended in HIV-infected pregnant patients (see CLINICAL PHARMACOLOGY, Pregnant Patients).
- Antiretroviral Pregnancy Registry
To monitor maternal-fetal outcomes of pregnant patients exposed to CRIXIVAN, an Antiretroviral Pregnancy Registry has been established. Physicians are encouraged to register patients by calling 1-800-258-4263.
- Nursing Mothers
Studies in lactating rats have demonstrated that indinavir is excreted in milk. Although it is not known whether CRIXIVAN is excreted in human milk, there exists the potential for adverse effects from indinavir in nursing infants. Mothers should be instructed to discontinue nursing if they are receiving CRIXIVAN. This is consistent with the recommendation by the U.S. Public Health Service Centers for Disease Control and Prevention that HIV-infected mothers not breast-feed their infants to avoid risking postnatal transmission of HIV.
- Pediatric Use
The optimal dosing regimen for use of indinavir in pediatric patients has not been established. A dose of 500 mg/m2 every eight hours has been studied in uncontrolled studies of 70 children, 3 to 18 years of age. The pharmacokinetic profiles of indinavir at this dose were not comparable to profiles previously observed in adults receiving the recommended dose (see CLINICAL PHARMACOLOGY, Pediatric). Although viral suppression was observed in some of the 32 children who were followed on this regimen through 24 weeks, a substantially higher rate of nephrolithiasis was reported when compared to adult historical data (see WARNINGS, Nephrolithiasis/Urolithiasis). Physicians considering the use of indinavir in pediatric patients without other protease inhibitor options should be aware of the limited data available in this population and the increased risk of nephrolithiasis.
- Geriatric Use
Clinical studies of CRIXIVAN did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal or cardiac function and of concomitant disease or other drug therapy.[1]
References
- ↑ 1.0 1.1 "CRIXIVAN (INDINAVIR SULFATE) CAPSULE [MERCK SHARP & DOHME CORP.]". Text " accessdate" ignored (help)
Adapted from the FDA Package Insert.