Hexachlorophene
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Hexachlorophene is an antibacterial cleansing agent that is FDA approved for the procedure of surgical scrubing and a bacteriostatic skin cleanser. It may also be used to control an outbreak of gram-positive infection where other infection control procedures have been unsuccessful. Use only as long as necessary for infection control. Common adverse reactions include dermatitis, photosensitivity.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- 1.- Wet hands and forearms with water. Apply approximately 5 mL of Hexachlorophene over the hands and rub into a copious lather by adding small amounts of water. Spread suds over hands and forearms and scrub well with a wet brush for 3 minutes. Pay particular attention to the nails and inter-digital spaces. A separate nail cleaner may be used. Rinse thoroughly under running water.
- 2.- Apply 5 mL of Hexachlorophene to hands again and scrub as above for another 3 minutes. Rinse thoroughly with running water and dry.
- 3.- For repeat surgical scrubs during the day, scrub thoroughly with the same amount of Hexachlorophene for 3 minutes only. Rinse thoroughly with water and dry.
- Wet hands with water. Dispense approximately 5 mL of Hexachlorophene into the palm, work up a lather with water and apply to area to be cleansed. Rinse thoroughly after each washing.
- Hexachlorophene must not be used for bathing infants. Infants may absorb the active compound in Hexachlorophene more readily than older children and adults. Such absorption has been associated with central nervous system effects such as convulsions.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Hexachlorophene in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Hexachlorophene in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Hexachlorophene FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Hexachlorophene in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Hexachlorophene in pediatric patients.
Contraindications
- Hexachlorophene must not be used on burned or denuded skin.
- Hexachlorophene must not be used for bathing infants. Infants may absorb the active compound in Hexachlorophene more readily than older children and adults. Such absorption has been associated with central nervous system effects such as convulsions.
- It must not be used as an occlusive dressing, wetpack, or lotion. It must not be used routinely for prophylactic total body bathing.
- It must not be used as a vaginal pack or tampon, or on any mucous membranes.
- Hexachlorophene must not be used on persons with sensitivity to any of its components. It must not be used on persons who have demonstrated primary light sensitivity to halogenated phenol derivatives because of the possibility of cross-sensitivity to hexachlorophene.
Warnings
RINSE THOROUGHLY AFTER EACH USE
- Patients should be closely monitored and use should be immediately discontinued at the first sign of any of the symptoms described below.
- Rapid absorption of hexachlorophene may occur with resultant toxic blood levels when preparations containing hexachlorophene are applied to skin lesions such as ichthyosis congenita, the dermatitis of Letterer-Siwe's syndrome, or other generalized dermatological conditions. Application to burns has also produced neurotoxicity and death.
Hexachlorophene SHOULD BE DISCONTINUED PROMPTLY IF SIGNS OR SYMPTOMS OF CEREBRAL IRRITABILITY OCCUR.
- Infants, especially premature infants or those with dermatoses, are particularly susceptible to hexachlorophene absorption. Systemic toxicity may be manifested by signs of stimulation (irritation) of the central nervous system, sometimes with convulsions.
- Infants have developed dermatitis, irritability, generalized clonic muscular contractions and decerebrate rigidity following application of a 6 percent hexachlorophene powder. Examination of brainstems of those infants revealed vacuolization like that which can be produced in newborn experimental animals following repeated topical application of 3 percent hexachlorophene. Moreover, a study of histologic sections of premature infants who died of unrelated causes has shown a positive correlation between hexachlorophene baths and lesions in white matter of brains.
Adverse Reactions
Clinical Trials Experience
- Adverse reactions to Hexachlorophene may include dermatitis and photosensitivity. Sensitivity to hexachlorophene is rare; however, persons who have developed photoallergy to similar compounds also may become sensitive to hexachlorophene.
- In persons with highly sensitive skin the use of Hexachlorophene may at times produce a reaction characterized by redness and/or mild scaling or dryness, especially when it is combined with such mechanical factors as excessive rubbing or exposure to heat or cold.
Postmarketing Experience
There is limited information regarding Hexachlorophene Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Hexachlorophene Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- There are no adequate and well-controlled studies in pregnant women. Hexachlorophene should be used during pregnancy only if the potential benefit justifies potential risk to the fetus.
- Hexachlorophene has been shown to be teratogenic and embryotoxic in rats when given by mouth or instilled into the vagina in large doses.
- Administration of 500 mg/kg diet or 20 to 30 mg/kg bw/day by gavage to rats caused some malformations (angulated ribs, cleft palate, micro- and anophthalmia) and reduction in litter size.
- Placental transfer and excretion in milk of hexachlorophene has been demonstrated in rats.
- In another study, doses of up to 50 mg/kg diet failed to produce any effects in 3 generations of rats. *Hexachlorophene did not interfere with reproduction in hamsters.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Hexachlorophene in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Hexachlorophene during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from hexachlorophene, a decision should be made whether to discontinue nursing or to discontinue the drug taking into account the importance of the drug to the mother.
Pediatric Use
- Hexachlorophene must not be used for bathing infants. Infants may absorb the active compound in Hexachlorophene more readily than older children and adults. Such absorption has been associated with central nervous system effects such as convuls
Geriatic Use
- Clinical studies of Hexachlorophene did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in response between the elderly and younger patients. In general, use in elderly patients should be cautious, reflecting the greater frequency of dermatological disease, peripheral circulatory disease, and decreased propensity for wound healing in this group. In addition, use in elderly patients should take into account any decreased hepatic, renal, or cardiac function, as well as any concomitant disease or other drug therapy.
Gender
There is no FDA guidance on the use of Hexachlorophene with respect to specific gender populations.
Race
There is no FDA guidance on the use of Hexachlorophene with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Hexachlorophene in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Hexachlorophene in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Hexachlorophene in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Hexachlorophene in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Hexachlorophene Administration in the drug label.
Monitoring
There is limited information regarding Hexachlorophene Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Hexachlorophene and IV administrations.
Overdosage
- The accidental ingestion of Hexachlorophene in amounts from 1 oz to 4 oz has caused anorexia, vomiting, abdominal cramps, diarrhea, dehydration, convulsions, hypotension, and shock, and in several reported instances, fatalities.
- If patients are seen early, the stomach should be evacuated by emesis or gastric lavage. Olive oil or vegetable oil (60 mL or 2 fl oz) may then be given to delay absorption of hexachlorophene, followed by a saline cathartic to hasten removal. Treatment is symptomatic and supportive; intravenous fluids (5 percent dextrose in physiologic saline solution) may be given for dehydration. Any other electrolyte derangement should be corrected. If marked hypotension occurs, vasopressor therapy is indicated. Use of opiates may be considered if gastrointestinal symptoms (cramping, diarrhea) are severe. Scheduled medical or surgical procedures should be postponed until the patient's condition has been evaluated and stabilized.
Pharmacology
Mechanism of Action
- Hexachlorophene is a bacteriostatic cleansing agent. It cleanses the skin thoroughly and has bacteriostatic action against staphylococci and other gram-positive bacteria. Cumulative antibacterial action develops with repeated use. Cleansing with alcohol or soaps containing alcohol removes the antibacterial residue.
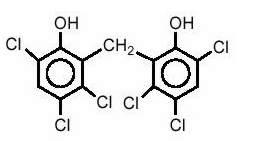
Structure
- Chemically, hexachlorophene is Phenol, 2,2'-methylenebis3,4,6-trichloro-; and has the following structural formula:
Pharmacodynamics
There is limited information regarding Hexachlorophene Pharmacodynamics in the drug label.
Pharmacokinetics
- Detectable blood levels of hexachlorophene following absorption through intact skin have been found in subjects who regularly scrubbed with hexachlorophene emulsion 3%.
- Hexachlorophene has the same slight acidity as normal skin (pH value 5.0 to 6.0).
Nonclinical Toxicology
There is limited information regarding Hexachlorophene Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Hexachlorophene Clinical Studies in the drug label.
How Supplied
- 5 oz plastic squeeze bottle (NDC 0024-1535-02).
- 1 pint plastic squeeze bottle (NDC 0024-1535-06).
Storage
- Store at room temperature up to 25° C (77° F)
Images
Drug Images
{{#ask: Page Name::Hexachlorophene |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Hexachlorophene |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Hexachlorophene Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Hexachlorophene interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Hexachlorophene Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.