Functional magnetic resonance imaging
Overview
Functional magnetic resonance imaging (fMRI) measures the haemodynamic response related to neural activity in the brain or spinal cord of humans or other animals. It is one of the most recently developed forms of neuroimaging.
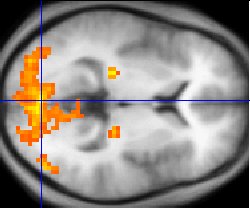
Background
Since the 1890s (Roy and Sherrington, 1890) it has been known that changes in blood flow and blood oxygenation in the brain (collectively known as hemodynamics) are closely linked to neural activity. When nerve cells are active they consume oxygen carried by hemoglobin in red blood cells from local capillaries. The local response to this oxygen utilization is an increase in blood flow to regions of increased neural activity, occurring after a delay of approximately 1-5 seconds. This hemodynamic response rises to a peak over 4-5 seconds, before falling back to baseline (and typically undershooting slightly). This leads to local changes in the relative concentration of oxyhemoglobin and deoxyhemoglobin and changes in local cerebral blood volume in addition to this change in local cerebral blood flow.
Blood-oxygen-level dependent
Blood-oxygen-level dependent or BOLD fMRI is a method of observing which areas of the brain are active at any given time. It was found by Dr. Seiji Ogawa[1] and Dr. Robert Turner, working independently, in 1990. Neurons do not have internal reserves of energy in the form of sugar and oxygen, so their firing causes a need for more energy to be brought in quickly. Through a process called the hemodynamic response, blood releases oxygen to them at a greater rate than to inactive neurons, and the difference in magnetic susceptibility between oxyhemoglobin and deoxyhemoglobin, and thus oxygenated or deoxygenated blood, leads to magnetic signal variation which can be detected using an MRI scanner. Given many repetitions of a thought, action or experience, statistical methods can be used to determine the areas of the brain which reliably have more of this difference as a result, and therefore which areas of the brain are active during that thought, action or experience.
Almost all fMRI research uses BOLD as the method for determining where activity occurs in the brain as the result of various experiences, but because the signals are relative and not individually quantitative, some question its rigor. Other methods which propose to measure neural activity directly have been attempted (for example, measurement of the Oxygen Extraction Fraction, or OEF, in regions of the brain, which measures how much of the oxyhemoglobin in the blood has been converted to deoxyhemoglobin[2]), but because the electromagnetic fields created by an active or firing neuron are so weak, the signal-to-noise ratio is extremely low and statistical methods used to extract quantitative data have been largely unsuccessful as of yet.
Hemoglobin is diamagnetic when oxygenated but paramagnetic when deoxygenated. The magnetic resonance (MR) signal of blood is therefore slightly different depending on the level of oxygenation. These differential signals can be detected using an appropriate MR pulse sequence as blood-oxygen-level dependent (BOLD) contrast. Higher BOLD signal intensities arise from increases in the concentration of oxygenated hemoglobin since the blood magnetic susceptibility now more closely matches the tissue magnetic susceptibility. By collecting data in an MRI scanner with parameters sensitive to changes in magnetic susceptibility one can assess changes in BOLD contrast. These changes can be either positive or negative depending upon the relative changes in both cerebral blood flow (CBF) and oxygen consumption. Increases in CBF that outstrip changes in oxygen consumption will lead to increased BOLD signal, conversely decreases in CBF that outstrip changes in oxygen consumption will cause decreased BOLD signal intensity.
Neural correlates of BOLD
The precise relationship between neural signals and BOLD is under active research. In general, changes in BOLD signal are well correlated with changes in blood flow. Numerous studies during the past several decades have identified a coupling between blood flow and metabolic rate; that is, the blood supply is tightly regulated in space and time to provide the nutrients for brain metabolism. However, neuroscientists have been seeking a more direct relationship between the blood supply and the neural inputs/outputs that can be related to observable electrical activity and circuit models of brain function.
While current data indicate that local field potentials, an index of integrated electrical activity, form a marginally better correlation with blood flow than the spiking action potentials that are most directly associated with neural communication, no simple measure of electrical activity to date has provided an adequate correlation with metabolism and the blood supply across a wide dynamic range. Presumably, this reflects the complex nature of metabolic processes, which form a superset with regards to electrical activity. Some recent results have suggested that the increase in cerebral blood flow (CBF) following neural activity is not causally related to the metabolic demands of the brain region, but rather is driven by the presence of neurotransmitters, especially glutamate.
Some other recent results suggest that an initial small, negative dip before the main positive BOLD signal is more highly localized and also correlates with measured local decreases in tissue oxygen concentration (perhaps reflecting increased local metabolism during neuron activation). Use of this more localized negative BOLD signal has enabled imaging of human ocular dominance columns in primary visual cortex, with resolution of about 0.5 mm. One problem with this technique is that the early negative BOLD signal is small and can only be seen using larger scanners with magnetic fields of at least 3 Tesla. Further, the signal is much smaller than the normal BOLD signal, making extraction of the signal from noise that much more difficult. Also, this initial dip occurs within 1-2 seconds of stimulus initiation, which may not be captured when signals are recorded at long repetition (TR). If the TR is sufficiently low, increased speed of the cerebral blood flow response due to consumption of vasoactive drugs (such as caffeine[3]) or natural differences in vascular responsivnesses may further obscure observation of the initial dip.
The BOLD signal is composed of CBF contributions from larger arteries and veins, smaller arterioles and venules, and capillaries. Experimental results indicate that the BOLD signal can be weighted to the smaller vessels, and hence closer to the active neurons, by using larger magnetic fields. For example, whereas about 70% of the BOLD signal arises from larger vessels in a 1.5 tesla scanner, about 70% arises from smaller vessels in a 4 tesla scanner. Furthermore, the size of the BOLD signal increases roughly as the square of the magnetic field strength. Hence there has been a push for larger field scanners to both improve localization and increase the signal. A few 7 tesla commercial scanners have become operational, and experimental 8 and 9 tesla scanners are under development.


Technique
BOLD effects are measured using rapid volumetric acquisition of images with contrast weighed by T2 or T2* (see MRI). Such images can be acquired with moderately good spatial and temporal resolution; images are usually taken every 1–4 seconds, and the voxels in the resulting image typically represent cubes of tissue about 2–4 millimeters on each side in humans. Recent technical advancements, such as the use of high magnetic fields and advanced "multichannel" RF reception, have advanced spatial resolution to the millimeter scale. Although responses to stimuli presented as close together as one or two seconds can be distinguished from one another, using a method known as event-related fMRI, the full time course of a BOLD response to a briefly presented stimulus lasts about 15 seconds for the robust positive response.
fMRI studies draw from many disciplines
To use fMRI effectively, an investigator must have a firm grasp of the relevant principles from all of these fields:
- Physics: Researchers should have a reasonable understanding of the physical principles underlying fMRI.
- Psychology: Almost all fMRI studies are essentially cognitive psychological, cognitive psychophysiological, and/or psychophysical experiments in which the MRI scanner is used to obtain an extra set of measurements in addition to behavioral and electroencephalographic measurements. This allows for more detailed theory testing and inference on perceptual and cognitive processes, and allows to relate these to specific brain structures.
- Neuroanatomy: The fMRI signals can be put into the context of previous knowledge only with an understanding of the neuroanatomy. Ultimately, the goal of all functional imaging experiments is to explain human cognition and behavior in terms of physical (anatomical) mechanisms.
- Statistics: Correct application of statistics is essential to "tease out" observations and avoid false-positive results.
- Electrophysiology: Familiarity with neuronal behavior at the electrophysiological level can help investigators design a useful fMRI study.
Seiji Ogawa and Kenneth Kwong are generally credited as the discoverers of the BOLD effect that underlies conventional fMRI.
Advantages and Disadvantages of fMRI
Like any technique, fMRI has advantages and disadvantages, and in order to be useful, the experiments that employ it must be carefully designed and conducted to maximize its strengths and minimize its weaknesses.
General disadvantages of the method
- The BOLD signal is only an indirect measure of neural activity, and is therefore susceptible to influence by non-neural changes in the body.
- BOLD signals are most strongly associated with the input to a given area than with the output. It is therefore possible (although unlikely) that a BOLD signal could be present in a given area even if there is no single unit activity.[4]
- Different brain areas have different hemodynamic responses, which would not be accurately reflected by the general linear model often used to filter fMRI time signals.
- For a non-invasive scan, fMRI has moderately good spatial resolution. However, the temporal response of the blood supply, which is the basis of fMRI, is poor relative to the electrical signals that define neuronal communication. Therefore, some research groups are working around this issue by combining fMRI with data collection techniques such as electroencephalography (EEG) or magnetoencephalography (MEG). EEG has much higher temporal resolution but rather poor spatial resolution, whereas MEG has much higher temporal resolution and similar spatial resolution. This has led some to suggest MEG is a more valuable tool than fMRI.
- fMRI has often been used to show activation localized to specific regions, thus minimizing the distributed nature of processing in neural networks. Several recent multivariate statistical techniques work around this issue by characterizing interactions between "active" regions found via traditional univariate techniques. Such techniques might prove useful in the future.
- fMRI is usually used to try to determine "where" task-related activity occurs in the brain. This has led to the charge that it is simply a modern-day phrenology. Some scientists prefer models which explain "how" psychological mechanisms function. The counter-argument to this criticism is that knowing "where" a cognitive function is located is vitally important. Neuropsychology, neurophysiology, and functional imaging each give us different windows of understanding into what each brain region does and how. The analogy to phrenology is somewhat misleading: phrenology has little or no basis in the scientific method, whereas fMRI permits hypotheses to be tested and strong inferences to be made.
- Many theoretical models used to explain fMRI signals are so poorly specified that they are not falsifiable (a central tenet from the scientific method). Hence, some argue, fMRI is not really a "science." The counter-argument is that an fMRI study can provide evidence to falsify a prior theory if it is well-designed. Also, well-specified mathematical and computational models of the neural processes underlying fMRI can make theories more concrete, allowing them to make predictions that can be verified or falsified by fMRI.
Advantages of fMRI
- It can noninvasively record brain signals (of humans and other animals) without risks of radiation inherent in other scanning methods, such as CT scans.
- It can record on a spatial resolution in the region of 3-6 millimeters, but with relatively poor temporal resolution (on the order of seconds) compared with techniques such as EEG. However, this is mainly because of the phenomena being measured, not because of the technique. EEG measures electrical/neural activity while fMRI measures blood activity, which has a longer response. The MRI equipment used for fMRI can be used for high temporal resolution, if one measures different phenomena.
General counterargument
Like any other technique, fMRI is as worthwhile as the design of the experiment using it. Many investigators have used fMRI ineffectively because they were not familiar with all aspects of the technique, or because they received their academic training in disciplines characterized by less rigor than some other branches of psychology and neuroscience. Ineffective use of the technique is a problem for the field, but it is not a consequence of the technique itself.
While the mechanistic information provided by fMRI is limited relative to classical techniques of electrophysiology and molecular biology, this is a general criticism of systems-level biology based upon changes in metabolism, blood supply, or ensemble indices of electrical activity. Most researchers believe that both "bottom-up" and "top-down" measurements are needed to inform our understanding of the complex mechanisms that transpose neural activity into behavior.
Commercial use
Omneuron [1] is a US-based company founded by Christopher deCharms that is researching potential practical and clinical applications of real time fMRI.
Applied fMRI Institute [2] is a San Diego, CA based company offering commercial use of their Siemens 3T TIM Trio.
Neurognostics [3] is a US-based company that offers a standardized fMRI system
Imagilys [4] is a European company specialized in clinical and research fMRI.
At least two companies have been set up to use fMRI in lie detection. They are No Lie MRI, Inc [5] and Cephos Corporation [6]. In episode 109 of the popular science show Mythbusters, the three members of the build team attempted to fool an FMRI test. Although two of them were unsuccessful, the third was able to successfully fool the machine.
The signals are extrapolated from the fMRI machine onto a screen, displaying the active regions of the brain. Depending on what regions are the most active, the technician can determine whether a subject is telling the truth or not. This technology is in its early stages of development, and many of its proponents hope to replace older lie detection techniques.
Scanning in practice

Subjects participating in a fMRI experiment are asked to lie still and are usually restrained with soft pads to prevent small motions from disturbing measurements. Some labs also employ bite bars to reduce motion, although these are unpopular as they can cause some discomfort to subjects. It is possible to correct for some amount of head movement with post-processing of the data, but large transient motion can render these attempts futile. Generally motion in excess of 3 millimeters will result in unusable data. The issue of motion is present for all populations, but most notably within populations that are not physically or emotionally equipped for even short MRI sessions (e.g., those with Alzheimer's Disease or schizophrenia, or young children). In these populations, various and negative reinforcement strategies can be employed in an attempt to attenuate motion artifacts, but in general the solution lies in designing a compatible paradigm with these populations.
An fMRI experiment usually lasts between 15 minutes and 2 hours. Depending on the purpose of study, subjects may view movies, hear sounds, smell odors, perform cognitive tasks such as memorization or imagination, press a few buttons, or perform other tasks. Researchers are required to give detailed instructions and descriptions of the experiment plan to each subject, who must sign a consent form before the experiment.
Safety is a very important issue in all experiments involving MRI. Potential subjects must ensure that they are able to enter the MRI environment. Due to the nature of the MRI scanner, there is an extremely strong magnetic field surrounding the MRI scanner (at least 1.5 teslas, possibly stronger). Potential subjects must be thoroughly examined for any ferromagnetic objects (e.g. watches, glasses, hair pins, pacemakers, bone plates and screws, etc.) before entering the scanning environment.
Related techniques
Aside from fMRI, there are other related ways to probe brain activity using magnetic resonance properties:
Contrast MR
An injected contrast agent such as an iron oxide that has been coated by a sugar or starch (to hide from the body's defense system), causes a local disturbance in the magnetic field that is measurable by the MRI scanner. The signals associated with these kinds of contrast agents are proportional to the cerebral blood volume. While this semi-invasive method presents a considerable disadvantage in terms of studying brain function in normal subjects, it enables far greater detection sensitivity than BOLD signal, which may increase the viability of fMRI in clinical populations. Other methods of investigating blood volume that do not require an injection are a subject of current research, although no alternative technique in theory can match the high sensitivity provided by injection of contrast agent.
Arterial spin labeling
By magnetic labeling the proximal blood supply using "arterial spin labeling" ASL, the associated signal is proportional to the cerebral blood flow, or perfusion. This method provides more quantitative physiological information than BOLD signal, and has the same sensitivity for detecting task-induced changes in local brain function
Magnetic resonance spectroscopic imaging
Magnetic resonance spectroscopic imaging (MRS) is another, NMR-based process for assessing function within the living brain. MRS takes advantage of the fact that protons (hydrogen atoms) residing in differing chemical environments depending upon the molecule they inhabit (H2O vs. protein, for example) possess slightly different resonant properties. For a given volume of brain (typically > 1 cubic cm), the distribution of these H resonances can be displayed as a spectrum.
The area under the peak for each resonance provides a quantitative measure of the relative abundance of that compound. The largest peak is composed of H2O. However, there are also discernible peaks for choline, creatine, N-acetylaspartate (NAA) and lactate. Fortuitously, NAA is mostly inactive within the neuron, serving as a precursor to glutamate and as storage for acetyl groups (to be used in fatty acid synthesis) — but its relative levels are a reasonable approximation of neuronal integrity and functional status. Brain diseases (schizophrenia, stroke, certain tumors, multiple sclerosis) can be characterized by the regional alteration in NAA levels when compared to healthy subjects. Creatine is used as a relative control value since its levels remain fairly constant, while choline and lactate levels have been used to evaluate brain tumors.
Diffusion tensor imaging
Diffusion tensor imaging (DTI) is a related use of MR to measure anatomical connectivity between areas. Although it is not strictly a functional imaging technique because it does not measure dynamic changes in brain function, the measures of inter-area connectivity it provides are complementary to images of cortical function provided by BOLD fMRI. White matter bundles carry functional information between brain regions. The diffusion of water molecules is hindered across the axes of these bundles, such that measurements of water diffusion can reveal information about the location of large white matter pathways. Illnesses that disrupt the normal organization or integrity of cerebral white matter (such as multiple sclerosis) have a quantitative impact on DTI measures.
Approaches to fMRI data analysis
The ultimate goal of fMRI data analysis is to detect correlations between brain activation and the task the subject performs during the scan. The BOLD signature of activation is relatively weak, however, so other sources of noise in the acquired data must be carefully controlled. This means that a series of processing steps must be performed on the acquired images before the actual statistical search for task-related activation can begin.
For a typical fMRI scan, the 3D volume of the subject's head is imaged every one or two seconds, producing a few hundred to a few thousand complete images per scanning session. The nature of MRI is such that these images are acquired in Fourier transform space, so they must be transformed back to image space to be useful. Because of practical limitations of the scanner the Fourier samples are not acquired on a grid, and scanner imperfections like thermal drift and spike noise introduce additional distortions. Small motions on the part of the subject and the subject's pulse and respiration will also affect the images.
The most common situation is that the researcher uses a pulse sequence supplied by the scanner vendor, such as an echo-planar imaging (EPI) sequence that allows for relatively rapid acquisition of many images. Software in the scanner platform itself then performs the reconstruction of images from Fourier transform space. During this stage some information is lost (specifically the complex phase of the reconstructed signal). Some types of artifacts, for example spike noise, become more difficult to remove after reconstruction, but if the scanner is working well these artifacts are thought to be relatively unimportant. For pulse sequences not provided by the vendor, for example spiral EPI, reconstruction must be done by software running on a separate platform.
After reconstruction the output of the scanning session consists of a series of 3D images of the brain. The most common corrections performed on these images are motion correction and correction for physiological effects. Outlier correction and spatial and/or temporal filtering may also be performed. If the task performed by the subject is thought to produce bursts of activation which are short compared to the BOLD response time (on the order of 6 seconds), temporal filtering may be performed at this stage to attempt to deconvolve out the BOLD response and recover the temporal pattern of activation.
At this point the data provides a time series of samples for each voxel in the scanned volume. A variety of methods are used to correlate these voxel time series with the task in order to produce maps of task-dependent activation.
Some fMRI neuroimaging software:
- AFNI [7]
- BrainVoyager [8]
- CamBA [9]
- Fiasco/FIAT [10]
- FreeSurfer [11]
- mrVista[12]
- FSL [13]
- SPM [14]
- AutoSPM: Automated SPM for Surgical Planning
- BioImage Suite
- nordicICE
See also
- Brain Mapping
- Brain function
- Event related fMRI
- Spinal fMRI
- SEEP fMRI
- EEG-fMRI
- Real-time fMRI
- Functional neuroimaging
- The fMRI Data Centre
- Linear transform model
References
- ↑ Ogawa, S., Lee, T.M., Nayak, A.S., and Glynn, P. (1990). Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med 14, 68-78
- ↑ Theory of NMR signal behavior in magnetically inho...[Magn Reson Med. 1994] - PubMed Result
- ↑ Behzadi, Y.; et al. (2006). "Caffeine reduces the initial dip in the visual bold response at 3 t.". Neuroimage. 32: 9–15. doi:10.1016/j.neuroimage.2006.03.005.
- ↑ Logothetis, N.K. (2001). "Neurophysiological investigation of the basis of the fMRI signal" (PDF). Nature. 412: 150. doi:10.1038/35084005.
Textbooks
Scott A. Huettel, Allen W. Song, Gregory McCarthy, Functional Magnetic Resonance Imaging, Sinauer Associates, 2004, ISBN 0-87893-288-7
Richard B. Buxton, An Introduction to Functional Magnetic Resonance Imaging: Principles and Techniques, Cambridge Univ Press, 2002, ISBN 0-52158-113-3
Journal articles
Weiller C; et al. (2006). "Role of functional imaging in neurological disorders". Journal of Magnetic Resonance Imaging. 23 (6): 840–850.
Lin, Lyons, and Berkowitz (2007). "Somatotopic Identification of Language-SMA in Language Processing via fMRI". Journal of Scientific and Practical Computing. 1 (2): 3–8. [15]
External links
- Rochester Center for Brain Imaging at the University of Rochester which has a 3T scanner open for research purposes
- Department of Biophysics at Medical College of Wisconsin
- Laboratory of Neuro Imaging at UCLA
- Athinoula A. Martinos Center for Biomedical Imaging [16] at Massachusetts General Hospital, which develops and supports FreeSurfer
- RadiologyInfo- The radiology information resource for patients: Functional Magnetic Resonance Imaging of the Brain
- The fMRI Data Center (fMRIDC) at Dartmouth College
- The Functional Imaging Laboratory at University College London
- The Centre for Functional Magnetic Resonance Imaging of the Brain at Oxford University
- Sir Peter Mansfield Magnetic Resonance Centre, University of Nottingham
- The Brain Mapping Unit (BMU), University of Cambridge
- About fMRI from Functional MRI Research Center, Columbia University
- Introduction to FMRI from the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain, Oxford University
- FMRLAB Toolbox for fMRI data analysis
- BrainMapping.ORG project Community web site for information Brain Mapping and methods
- York Neuroimaging Center at University of York
- fMRI.pl Functional Imaging Lab at Warsaw University of Technology
- Functional Neuroimaging Lab at University of Sao Paulo - Ribeirao Preto - Brazil
- A list of the best introductions to fMRI on the web
- fMRI Methods Wiki - with advice on how to report on fMRI studies
- fMRI entry at Scholarpedia
Notes
de:Funktionelle Magnetresonanztomographie el:Λειτουργική Απεικόνιση Μαγνητικού Συντονισμού is:Starfræn segulómmyndun it:Risonanza magnetica funzionale hu:Funkcionális mágneses rezonancia-vizsgálat nl:Functionele MRI no:Funksjonell Magnetresonanstomografi