Drospirenone and Ethinyl estradiol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Drospirenone and Ethinyl estradiol is a contraceptive agent that is FDA approved for the treatment of premenstrual dysphoric disorder (PMDD), acne, and contraception. Common adverse reactions include headache/migraine, menstrual irregularities, nausea/vomiting, breast pain/tenderness and mood changes.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Acne vulgaris (Moderate)
- Nikki (drospirenone and ethinyl estradiol tablets USP), 3 mg/0.02 mg is indicated for the treatment of moderate acne vulgaris in women at least 14 years of age, who have no known contraindications to oral contraceptive therapy and have achieved menarche. Nikki (drospirenone and ethinyl estradiol tablets USP), 3 mg/0.02 mg should be used for the treatment of acne only if the patient desires an oral contraceptive for birth control.
- Dosing information
- Yaz(R), 1 active (pink) tablet ORALLY every day for 24 consecutive days followed by 1 inert (white) tablet daily for 4 days per menstrual cycle
- Yaz(R), begin therapy either on the first day of menstrual period or on the first Sunday after the onset of menstruation
- Yaz(R), begin next and all subsequent 28-day regimens of therapy on the same day of the week as the first regimen began and follow the same schedule
Contraception
- Nikki™ (drospirenone and ethinyl estradiol tablets USP), 3 mg/0.02 mg is indicated for use by women to prevent pregnancy.
- Dosing information
- How to Take Nikki
- Take one tablet by mouth at the same time every day. The failure rate may increase when pills are missed or taken incorrectly.
- To achieve maximum contraceptive effectiveness, Nikki must be taken exactly as directed, in the order directed on the wallet. Single missed pills should be taken as soon as remembered.
- How to Start Nikki
- Instruct the patient to begin taking Nikki either on the first day of her menstrual period (Day 1 Start) or on the first Sunday after the onset of her menstrual period (Sunday Start).
- Day 1 Start:
- During the first cycle of Nikki use, instruct the patient to take one pink Nikki daily, beginning on Day 1 of her menstrual cycle. (The first day of menstruation is Day 1.) She should take one pink Nikki daily for 24 consecutive days, followed by one white inert tablet daily on Days 25 through 28. Nikki should be taken in the order directed on the package at the same time each day, preferably after the evening meal or at bedtime with some liquid, as needed. Nikki can be taken without regard to meals. If Nikki is first taken later than the first day of the menstrual cycle, Nikki should not be considered effective as a contraceptive until after the first 7 consecutive days of product administration. Instruct the patient to use a non-hormonal contraceptive as back-up during the first 7 days. The possibility of ovulation and conception prior to initiation of medication should be considered.
- Sunday Start:
- During the first cycle of Nikki use, instruct the patient to take one pink Nikki daily, beginning on the first Sunday after the onset of her menstrual period. She should take one pink Nikki daily for 24 consecutive days, followed by one white inert tablet daily on Days 25 through 28. Nikki should be taken in the order directed on the package at the same time each day, preferably after the evening meal or at bedtime with some liquid, as needed. Nikki can be taken without regard to meals. Nikki should not be considered effective as a contraceptive until after the first 7 consecutive days of product administration. Instruct the patient to use a non-hormonal contraceptive as back-up during the first 7 days. The possibility of ovulation and conception prior to initiation of medication should be considered.
- The patient should begin her next and all subsequent 28-day regimens of Nikki on the same day of the week that she began her first regimen, following the same schedule. She should begin taking her pink tablets on the next day after ingestion of the last white tablet, regardless of whether or not a menstrual period has occurred or is still in progress. Anytime a subsequent cycle of Nikki is started later than the day following administration of the last white tablet, the patient should use another method of contraception until she has taken a pink Nikki daily for seven consecutive days.
- When switching from a different birth control pill:
- When switching from another birth control pill, Nikki should be started on the same day that a new pack of the previous oral contraceptive would have been started.
- When switching from a method other than a birth control pill:
- When switching from a transdermal patch or vaginal ring, Nikki should be started when the next application would have been due. When switching from an injection, Nikki should be started when the next dose would have been due. When switching from an intrauterine contraceptive or an implant, Nikki should be started on the day of removal.
- Withdrawal bleeding usually occurs within 3 days following the last pink tablet. If spotting or breakthrough bleeding occurs while taking Nikki, instruct the patient to continue taking Nikki by the regimen described above. Counsel her that this type of bleeding is usually transient and without significance; however, advise her that if the bleeding is persistent or prolonged, she should consult her healthcare provider.
- Although the occurrence of pregnancy is low if Nikki is taken according to directions, if withdrawal bleeding does not occur, consider the possibility of pregnancy. If the patient has not adhered to the prescribed dosing schedule (missed one or more active tablets or started taking them on a day later than she should have), consider the possibility of pregnancy at the time of the first missed period and take appropriate diagnostic measures. If the patient has adhered to the prescribed regimen and misses two consecutive periods, rule out pregnancy. Discontinue Nikki if pregnancy is confirmed.
- The risk of pregnancy increases with each active pink tablet missed. For additional patient instructions regarding missed pills, see the "What to Do if You Miss Pills" section in the FDA Approved Patient Labeling. If breakthrough bleeding occurs following missed tablets, it will usually be transient and of no consequence. If the patient misses one or more white tablets, she should still be protected against pregnancy provided she begins taking a new cycle of pink tablets on the proper day.
- For postpartum women who do not breastfeed or after a second trimester abortion, start Nikki no earlier than 4 weeks postpartum due to the increased risk of thromboembolism. If the patient starts on Nikki postpartum and has not yet had a period, evaluate for possible pregnancy, and instruct her to use an additional method of contraception until she has taken Nikki for 7 consecutive days.
- Advice in Case of Gastrointestinal Disturbances
Premenstrual dysphoric disorder
- Dosing information
- Yaz(R), 1 active (pink) tablet ORALLY every day for 24 consecutive days followed by 1 inert (white) tablet daily for 4 days per menstrual cycle
- Yaz(R), begin therapy either on the first day of menstrual period or on the first Sunday after the onset of menstruation
- Yaz(R), begin next and all subsequent 28-day regimens of therapy on the same day of the week as the first regimen began and follow the same schedule
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Drospirenone and Ethinyl estradiol in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Drospirenone and Ethinyl estradiol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Acne vulgaris (Moderate)
- Dosing information
- The recommended dose to treat moderate acne vulgaris in women at least 14 years of age is one active (pink) Yaz(R) tablet orally every day for 24 consecutive days, followed by one inert (white) tablet daily for 4 days per menstrual cycle. Therapy should start either on the first day of menstrual period or on the first Sunday after the onset of menstruation. Next or all subsequent 28-day regimens of therapy should be started on the same day of the week as the first regimen began and must follow the same schedule.
Contraception
- Dosing information
- How to Take Nikki
- Take one tablet by mouth at the same time every day. The failure rate may increase when pills are missed or taken incorrectly.
- To achieve maximum contraceptive effectiveness, Nikki must be taken exactly as directed, in the order directed on the wallet. Single missed pills should be taken as soon as remembered.
- How to Start Nikki
- Instruct the patient to begin taking Nikki either on the first day of her menstrual period (Day 1 Start) or on the first Sunday after the onset of her menstrual period (Sunday Start).
- Day 1 Start:
- During the first cycle of Nikki use, instruct the patient to take one pink Nikki daily, beginning on Day 1 of her menstrual cycle. (The first day of menstruation is Day 1.) She should take one pink Nikki daily for 24 consecutive days, followed by one white inert tablet daily on Days 25 through 28. Nikki should be taken in the order directed on the package at the same time each day, preferably after the evening meal or at bedtime with some liquid, as needed. Nikki can be taken without regard to meals. If Nikki is first taken later than the first day of the menstrual cycle, Nikki should not be considered effective as a contraceptive until after the first 7 consecutive days of product administration. Instruct the patient to use a non-hormonal contraceptive as back-up during the first 7 days. The possibility of ovulation and conception prior to initiation of medication should be considered.
- Sunday Start:
- During the first cycle of Nikki use, instruct the patient to take one pink Nikki daily, beginning on the first Sunday after the onset of her menstrual period. She should take one pink Nikki daily for 24 consecutive days, followed by one white inert tablet daily on Days 25 through 28. Nikki should be taken in the order directed on the package at the same time each day, preferably after the evening meal or at bedtime with some liquid, as needed. Nikki can be taken without regard to meals. Nikki should not be considered effective as a contraceptive until after the first 7 consecutive days of product administration. Instruct the patient to use a non-hormonal contraceptive as back-up during the first 7 days. The possibility of ovulation and conception prior to initiation of medication should be considered.
- The patient should begin her next and all subsequent 28-day regimens of Nikki on the same day of the week that she began her first regimen, following the same schedule. She should begin taking her pink tablets on the next day after ingestion of the last white tablet, regardless of whether or not a menstrual period has occurred or is still in progress. Anytime a subsequent cycle of Nikki is started later than the day following administration of the last white tablet, the patient should use another method of contraception until she has taken a pink Nikki daily for seven consecutive days.
- When switching from a different birth control pill:
- When switching from another birth control pill, Nikki should be started on the same day that a new pack of the previous oral contraceptive would have been started.
- When switching from a method other than a birth control pill:
- When switching from a transdermal patch or vaginal ring, Nikki should be started when the next application would have been due. When switching from an injection, Nikki should be started when the next dose would have been due. When switching from an intrauterine contraceptive or an implant, Nikki should be started on the day of removal.
- Withdrawal bleeding usually occurs within 3 days following the last pink tablet. If spotting or breakthrough bleeding occurs while taking Nikki, instruct the patient to continue taking Nikki by the regimen described above. Counsel her that this type of bleeding is usually transient and without significance; however, advise her that if the bleeding is persistent or prolonged, she should consult her healthcare provider.
- Although the occurrence of pregnancy is low if Nikki is taken according to directions, if withdrawal bleeding does not occur, consider the possibility of pregnancy. If the patient has not adhered to the prescribed dosing schedule (missed one or more active tablets or started taking them on a day later than she should have), consider the possibility of pregnancy at the time of the first missed period and take appropriate diagnostic measures. If the patient has adhered to the prescribed regimen and misses two consecutive periods, rule out pregnancy. Discontinue Nikki if pregnancy is confirmed.
- The risk of pregnancy increases with each active pink tablet missed. For additional patient instructions regarding missed pills, see the "What to Do if You Miss Pills" section in the FDA Approved Patient Labeling. If breakthrough bleeding occurs following missed tablets, it will usually be transient and of no consequence. If the patient misses one or more white tablets, she should still be protected against pregnancy provided she begins taking a new cycle of pink tablets on the proper day.
- For postpartum women who do not breastfeed or after a second trimester abortion, start Nikki no earlier than 4 weeks postpartum due to the increased risk of thromboembolism. If the patient starts on Nikki postpartum and has not yet had a period, evaluate for possible pregnancy, and instruct her to use an additional method of contraception until she has taken Nikki for 7 consecutive days.
- Advice in Case of Gastrointestinal Disturbances
- In case of severe vomiting or diarrhea, absorption may not be complete and additional contraceptive measures should be taken. If vomiting occurs within 3 to 4 hours after tablet-taking, this can be regarded as a missed tablet.
Premenstrual dysphoric disorder
- Dosing information
- The recommended dose to treat premenstrual dysphoric disorder in females who have achieved menarche is one active (pink) Yaz(R) tablet orally every day for 24 consecutive days, followed by one inert (white) tablet daily for 4 days per menstrual cycle. Therapy should start either on the first day of menstrual period or on the first Sunday after the onset of menstruation. Next or all subsequent 28-day regimens of therapy should be started on the same day of the week as the first regimen began and must follow the same schedule. Efficacy for premenstrual dysphoric disorder has not been assessed beyond 3 menstrual cycles.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Drospirenone and Ethinyl estradiol in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Drospirenone and Ethinyl estradiol in pediatric patients.
Contraindications
- Do not prescribe Nikki to women who are known to have the following:
- A high risk of arterial or venous thrombotic diseases. Examples include women who are known to:
- Smoke, if over age 35
- Have deep vein thrombosis or pulmonary embolism, now or in the past
- Have thrombogenic valvular or thrombogenic rhythm diseases of the heart (for example, subacute bacterial endocarditis with valvular disease, or atrial fibrillation)
- Have inherited or acquired hypercoagulopathies
- Have uncontrolled hypertension
- Have diabetes mellitus with vascular disease
- Have headaches with focal neurological symptoms or have migraine headaches with or without aura if over age 35
- Undiagnosed abnormal uterine bleeding
- Breast cancer or other estrogen- or progestin-sensitive cancer, now or in the past
- Liver tumors, benign or malignant, or liver disease
- Pregnancy, because there is no reason to use COCs during pregnancy
Warnings
Thromboembolic Disorders and Other Vascular Problems
- Stop Nikki if an arterial or venous thrombotic (VTE) event occurs.
- Based on presently available information on DRSP-containing COCs with 0.03 mg ethinyl estradiol (that is, Yasmin), DRSP-containing COCs may be associated with a higher risk of venous thromboembolism (VTE) than COCs containing the progestin levonorgestrel or some other progestins. Epidemiologic studies that compared the risk of VTE reported that the risk ranged from no increase to a three-fold increase. Before initiating use of Nikki in a new COC user or a woman who is switching from a contraceptive that does not contain DRSP, consider the risks and benefits of a DRSP-containing COC in light of her risk of a VTE. Known risk factors for VTE include smoking, obesity, and family history of VTE, in addition to other factors that contraindicate use of COCs.
- A number of studies have compared the risk of VTE for users of Yasmin (which contains 0.03 mg of EE and 3 mg of DRSP) to the risk for users of other COCs, including COCs containing levonorgestrel. Those that were required or sponsored by regulatory agencies are summarized in Table 1.
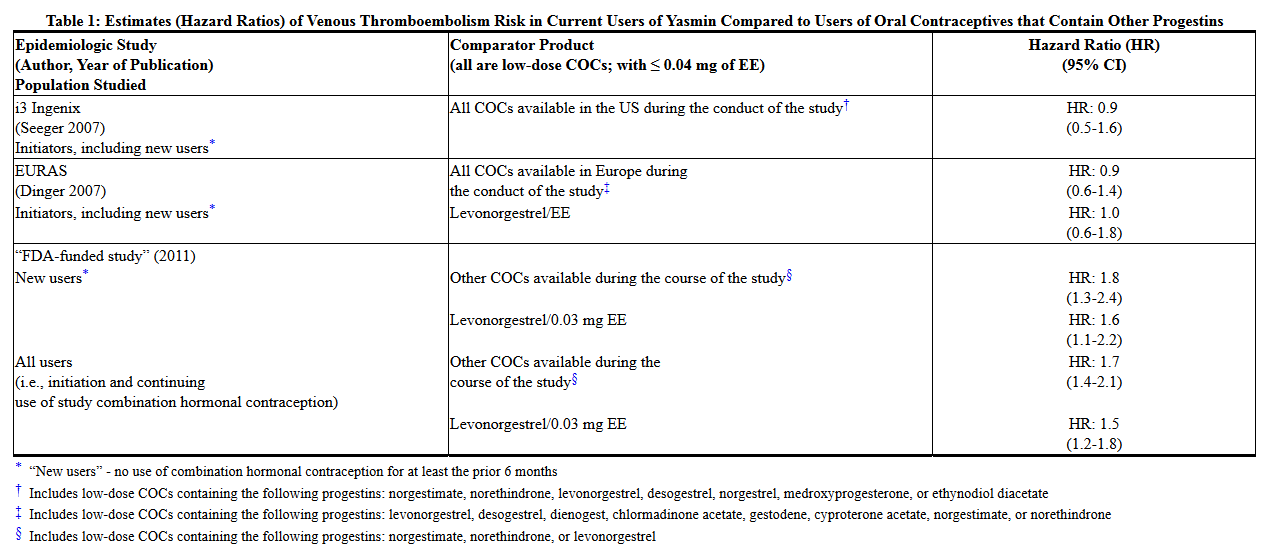
- In addition to these "regulatory studies," other studies of various designs have been conducted. Overall, there are two prospective cohort studies (see Table 1): the US post-approval safety study Ingenix [Seeger 2007], the European post-approval safety study EURAS (European Active Surveillance Study) [Dinger 2007]. An extension of the EURAS study, the Long-Term Active Surveillance Study (LASS), did not enroll additional subjects, but continued to assess VTE risk. There are three retrospective cohort studies: one study in the US funded by the FDA (see Table 1), and two from Denmark [Lidegaard 2009, Lidegaard 2011]. There are two case-control studies: the Dutch MEGA study analysis [van Hylckama Vlieg 2009] and the German case-control study [Dinger 2010]. There are two nested case-control studies that evaluated the risk of non-fatal idiopathic VTE: the PharMetrics study [Jick 2011] and the GPRD study [Parkin 2011]. The results of all of these studies are presented in Figure 1.
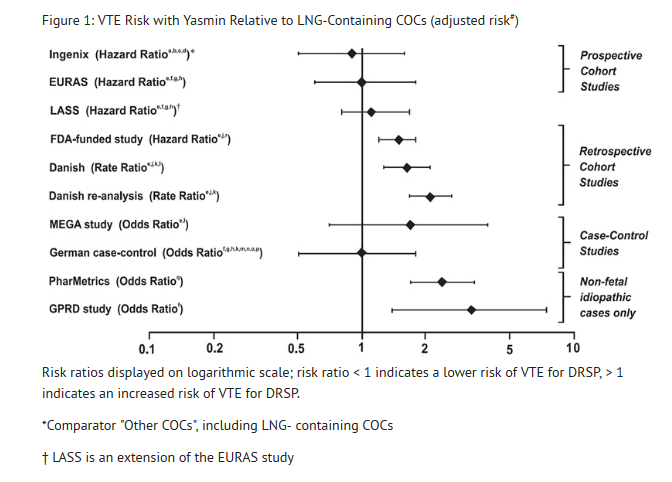
- Some adjustment factors are indicated by superscript letters: a) Current heavy smoking, b) hypertension, c) obesity, d) family history, e) age, f) BMI, g) duration of use, h) VTE history, i) period of inclusion, j) calendar year, k) education, l) length of use, m) parity, n) chronic disease, o) concomitant medication, p) smoking, q) duration of exposure, r) site
- (References: Ingenix [Seeger 2007]2, EURAS (European Active Surveillance Study) [Dinger 2007]3, LASS (Long-Term Active Surveillance Study) [Dinger, unpublished document on file], FDA-funded study [Sidney 2011]4, Danish [Lidegaard 2009]5, Danish reanalysis [Lidegaard 2011]6, MEGA study [van Hylckama Vlieg 2009]7, German Case-Control study [Dinger 2010]8, PharMetrics [Jick 2011]9, GPRD study [Parkin 2011]10)
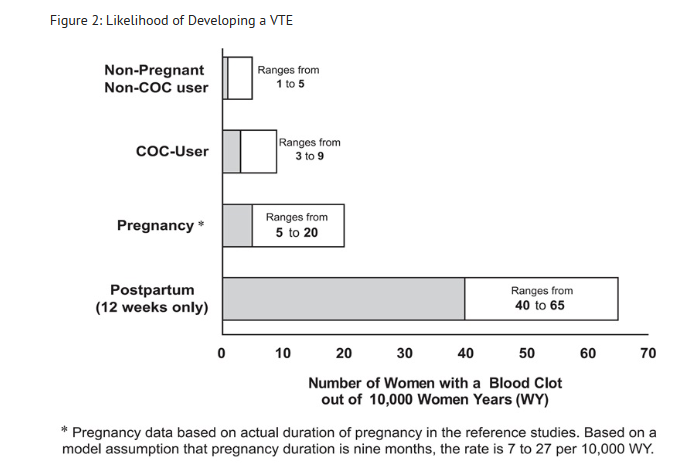
- Although the absolute VTE rates are increased for users of hormonal contraceptives compared to non-users, the rates during pregnancy are even greater, especially during the post-partum period (see Figure 2). The risk of VTE in women using COCs has been estimated to be 3 to 9 per 10,000 woman-years. The risk of VTE is highest during the first year of use. Data from a large, prospective cohort safety study of various COCs suggest that this increased risk, as compared to that in non-COC users, is greatest during the first 6 months of COC use. Data from this safety study indicate that the greatest risk of VTE is present after initially starting a COC or restarting (following a 4 week or greater pill-free interval) the same or a different COC.
- The risk of thromboembolic disease due to oral contraceptives gradually disappears after COC use is discontinued.
- Figure 2 shows the risk of developing a VTE for women who are not pregnant and do not use oral contraceptives, for women who use oral contraceptives, for pregnant women, and for women in the postpartum period. To put the risk of developing a VTE into perspective: If 10,000 women who are not pregnant and do not use oral contraceptives are followed for one year, between 1 and 5 of these women will develop a VTE.
- If feasible, stop Nikki at least 4 weeks before and through 2 weeks after major surgery or other surgeries known to have an elevated risk of thromboembolism.
- Start Nikki no earlier than 4 weeks after delivery, in women who are not breastfeeding. The risk of postpartum thromboembolism decreases after the third postpartum week, whereas the risk of ovulation increases after the third postpartum week.
- Use of COCs also increases the risk of arterial thromboses such as strokes and myocardial infarctions, especially in women with other risk factors for these events.
- COCs have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes), although, in general, the risk is greatest among older (>35 years of age), hypertensive women who also smoke. COCs also increase the risk for stroke in women with other underlying risk factors.
- Oral contraceptives must be used with caution in women with cardiovascular disease risk factors.
- Stop Nikki if there is unexplained loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions. Evaluate for retinal vein thrombosis immediately.
Hyperkalemia
- Nikki contains 3 mg of the progestin DRSP which has antimineralocorticoid activity, including the potential for hyperkalemia in high-risk patients, comparable to a 25 mg dose of spironolactone. Nikki should not be used in patients with conditions that predispose to hyperkalemia (that is, renal impairment, hepatic impairment and adrenal insufficiency). Women receiving daily, long-term treatment for chronic conditions or diseases with medications that may increase serum potassium concentration should have their serum potassium concentration checked during the first treatment cycle. Medications that may increase serum potassium concentration include ACE inhibitors, angiotensin-II receptor antagonists, potassium-sparing diuretics, potassium supplementation, heparin, aldosterone antagonists, and NSAIDS.
Carcinoma of the Breasts and Reproductive Organs
- Women who currently have or have had breast cancer should not use Nikki because breast cancer is a hormonally-sensitive tumor.
- There is substantial evidence that COCs do not increase the incidence of breast cancer. Although some past studies have suggested that COCs might increase the incidence of breast cancer, more recent studies have not confirmed such findings.
- Some studies suggest that COCs are associated with an increase in the risk of cervical cancer or intraepithelial neoplasia. However, there is controversy about the extent to which these findings may be due to differences in sexual behavior and other factors.
Liver Disease
- Discontinue Nikki if jaundice develops. Steroid hormones may be poorly metabolized in patients with impaired liver function. Acute or chronic disturbances of liver function may necessitate the discontinuation of COC use until markers of liver function return to normal and COC causation has been excluded.
- Hepatic adenomas are associated with COC use. An estimate of the attributable risk is 3.3 cases/100,000 COC users. Rupture of hepatic adenomas may cause death through intra-abdominal hemorrhage.
- Studies have shown an increased risk of developing hepatocellular carcinoma in long-term (>8 years) COC users. However, the attributable risk of liver cancers in COC users is less than one case per million users.
- Oral contraceptive-related cholestasis may occur in women with a history of pregnancy-related cholestasis. Women with a history of COC-related cholestasis may have the condition recur with subsequent COC use.
High Blood Pressure
- For women with well-controlled hypertension, monitor blood pressure and stop Nikki if blood pressure rises significantly. Women with uncontrolled hypertension or hypertension with vascular disease should not use COCs.
- An increase in blood pressure has been reported in women taking COCs, and this increase is more likely in older women and with extended duration of use. The incidence of hypertension increases with increasing concentration of progestin.
Gallbladder Disease
- Studies suggest a small increased relative risk of developing gallbladder disease among COC users.
Carbohydrate and Lipid Metabolic Effects
- Carefully monitor prediabetic and diabetic women who are taking Nikki. COCs may decrease glucose intolerance in a dose-related fashion.
- Consider alternative contraception for women with uncontrolled dyslipidemias. A small proportion of women will have adverse lipid changes while on COC's.
- Women with hypertriglyceridemia, or a family history thereof, may be at an increased risk of pancreatitis when using COCs.
Headache
- If a woman taking Nikki develops new headaches that are recurrent, persistent, or severe, evaluate the cause and discontinue Nikki if indicated.
- An increase in frequency or severity of migraine during COC use (which may be prodromal of a cerebrovascular event) may be a reason for immediate discontinuation of the COC.
Bleeding Irregularities
- Unscheduled (breakthrough or intracyclic) bleeding and spotting sometimes occur in patients on COCs, especially during the first three months of use. If bleeding persists or occurs after previously regular cycles, check for causes such as pregnancy or malignancy. If pathology and pregnancy are excluded, bleeding irregularities may resolve over time or with a change to a different COC.
- Based on patient diaries from two contraceptive clinical trials of Nikki, 8 to 25% of women experienced unscheduled bleeding per 28-day cycle. A total of 12 subjects out of 1,056 (1.1%) discontinued due to menstrual disorders including intermenstrual bleeding, menorrhagia, and metrorrhagia.
- Women who use Nikki may experience absence of withdrawal bleeding, even if they are not pregnant. Based on subject diaries from contraception trials for up to 13 cycles, 6 to 10% of women experienced cycles with no withdrawal bleeding. Some women may encounter post-pill amenorrhea or oligomenorrhea, especially when such a condition was pre-existent.
- If withdrawal bleeding does not occur, consider the possibility of pregnancy. If the patient has not adhered to the prescribed dosing schedule (missed one or more active tablets or started taking them on a day later than she should have), consider the possibility of pregnancy at the time of the first missed period and take appropriate diagnostic measures. If the patient has adhered to the prescribed regimen and misses two consecutive periods, rule out pregnancy.
COC Use Before or During Early Pregnancy
- Extensive epidemiological studies have revealed no increased risk of birth defects in women who have used oral contraceptives prior to pregnancy. Studies also do not suggest a teratogenic effect, particularly in so far as cardiac anomalies and limb-reduction defects are concerned, when taken inadvertently during early pregnancy.
- The administration of oral contraceptives to induce withdrawal bleeding should not be used as a test for pregnancy
Depression
- Women with a history of depression should be carefully observed and Nikki discontinued if depression recurs to a serious degree.
Interference with Laboratory Tests
- The use of COCs may change the results of some laboratory tests, such as coagulation factors, lipids, glucose tolerance, and binding proteins. Women on thyroid hormone replacement therapy may need increased doses of thyroid hormone because serum concentrations of thyroid-binding globulin increase with use of COCs.
- DRSP causes an increase in plasma renin activity and plasma aldosterone induced by its mild antimineralocorticoid activity.
Monitoring
- A woman who is taking COCs should have a yearly visit with her healthcare provider for a blood pressure check and for other indicated healthcare.
Other Conditions
- In women with hereditary angioedema, exogenous estrogens may induce or exacerbate symptoms of angioedema. Chloasma may occasionally occur, especially in women with a history of chloasma gravidarum. Women with a tendency to chloasma should avoid exposure to the sun or ultraviolet radiation while taking COCs.
Adverse Reactions
Clinical Trials Experience
- The following serious adverse reactions with the use of COCs are discussed elsewhere in the labeling:
- Serious cardiovascular events and stroke
- Vascular events
- Liver disease
- Adverse reactions commonly reported by COC users are:
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Contraception and Acne Clinical Trials
- The data provided reflect the experience with the use of Nikki in the adequate and well-controlled studies for contraception (N=1,056) and for moderate acne vulgaris (N=536).
- For contraception, a Phase 3, multicenter, multinational, open-label study was conducted to evaluate safety and efficacy up to one year in 1,027 women aged 17 - 36 who took at least one dose of Nikki. A second Phase 3 study was a single center, open-label, active-controlled study to evaluate the effect of 7 28-day cycles of Nikki on carbohydrate metabolism, lipids and hemostasis in 29 women aged 18-35. For acne, two multicenter, double-blind, randomized, placebo-controlled studies, in 536 women aged 14-45 with moderate acne vulgaris who took at least one dose of Nikki, evaluated the safety and efficacy during up to 6 cycles.
- The adverse reactions seen across the 2 indications overlapped, and are reported using the frequencies from the pooled dataset. The most common adverse reactions (> 2% of users) were: headache/migraine (6.7%), menstrual irregularities (including vaginal hemorrhage [primarily spotting] and metrorrhagia (4.7%), nausea/vomiting (4.2%), breast pain/tenderness (4%) and mood changes (mood swings, depression, depressed mood and affect lability) (2.2%).
Adverse Reactions (≥1%) Leading to Study Discontinuation
Contraception Clinical Trials:
- Of 1,056 women, 6.6% discontinued from the clinical trials due to an adverse reaction; the most frequent adverse reactions leading to discontinuation were headache/migraine (1.6%) and nausea/vomiting (1.0%).
Acne Clinical Trials:
- Of 536 women, 5.4% discontinued from the clinical trials due to an adverse reaction; the most frequent adverse reaction leading to discontinuation was menstrual irregularities (including menometrorrhagia, menorrhagia, metrorrhagia and vaginal hemorrhage) (2.2%).
Serious Adverse Reactions
- Contraception Clinical Trials: migraine and cervical dysplasia
- Acne Clinical Trials: none reported in the clinical trials
Postmarketing Experience
- The following adverse reactions have been identified during post approval use of Nikki. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Adverse reactions are grouped into System Organ Classes, and ordered by frequency.
Vascular Disorders
- Venous and arterial thromboembolic events (including pulmonary emboli, deep vein thrombosis, cerebral thrombosis, retinal thrombosis, myocardial infarction and stroke), hypertension (including hypertensive crisis)
Hepatobiliary Disorders
- Gallbladder disease, liver function disturbances, liver tumors
Immune System Disorders
- Hypersensitivity (including anaphylactic reaction)
Metabolism and Nutrition Disorders
- Hyperkalemia, hypertriglyceridemia, changes in glucose tolerance or effect on peripheral insulin resistance (including diabetes mellitus)
Skin and Subcutaneous Tissue Disorders
Gastrointestinal Disorders
Musculoskeletal and Connective Tissue Disorders
Drug Interactions
- Consult the labeling of all concurrently-used drugs to obtain further information about interactions with hormonal contraceptives or the potential for enzyme alterations.
Effects of Other Drugs on Combined Oral Contraceptives
Substances Diminishing the Efficacy of COCs
- Drugs or herbal products that induce certain enzymes, including cytochrome P450 3A4 (CYP3A4), may decrease the effectiveness of COCs or increase breakthrough bleeding. Some drugs or herbal products that may decrease the effectiveness of hormonal contraceptives include phenytoin, barbiturates, carbamazepine, bosentan, felbamate, griseofulvin, oxcarbazepine, rifampicin, topiramate and products containing St. John's wort. Interactions between oral contraceptives and other drugs may lead to breakthrough bleeding and/or contraceptive failure. Counsel women to use an alternative method of contraception or a back-up method when enzyme inducers are used with COCs, and to continue back-up contraception for 28 days after discontinuing the enzyme inducer to ensure contraceptive reliability.
Substances Increasing the Plasma Concentrations of COCs
- Co-administration of atorvastatin and certain COCs containing EE increase AUC values for EE by approximately 20%. Ascorbic acid and acetaminophen may increase plasma EE concentrations, possibly by inhibition of conjugation. CYP3A4 inhibitors such as itraconazole or ketoconazole may increase plasma hormone concentrations.
- Human immunodeficiency Virus (HIV)/ Hepatitis C Virus (HCV) Protease Inhibitors and Non-nucleoside Reverse Transcriptase Inhibitors:
- Significant changes (increase or decrease) in the plasma concentrations of estrogen and progestin have been noted in some cases of co-administration with HIV/HCV protease inhibitors or with non-nucleoside reverse transcriptase inhibitors.
- Antibiotics
- There have been reports of pregnancy while taking hormonal contraceptives and antibiotics, but clinical pharmacokinetic studies have not shown consistent effects of antibiotics on plasma concentrations of synthetic steroids.
Effect on DRSP
- The main metabolites of DRSP in human plasma are generated without involvement of the CYP system. Inhibitors of this enzyme system are therefore unlikely to influence the metabolism of DRSP.
Effects of Combined Oral Contraceptives on Other Drugs
- COCs containing EE may inhibit the metabolism of other compounds. COCs have been shown to significantly decrease plasma concentrations of lamotrigine, likely due to induction of lamotrigine glucuronidation. This may reduce seizure control; therefore, dosage adjustments of lamotrigine may be necessary. Consult the labeling of the concurrently-used drug to obtain further information about interactions with COCs or the potential for enzyme alterations.
- In vitro and clinical studies did not indicate an inhibitory potential of DRSP towards human CYP enzymes at clinically relevant concentrations.
- Women on thyroid hormone replacement therapy may need increased doses of thyroid hormone because serum concentration of thyroid-binding globulin increases with use of COCs.
Potential to Increase Serum Potassium Concentration
- There is a potential for an increase in serum potassium concentration in women taking Nikki with other drugs that may increase serum potassium.
Interference with Laboratory Tests
- The use of contraceptive steroids may influence the results of certain laboratory tests, such as coagulation factors, lipids, glucose tolerance, and binding proteins. DRSP causes an increase in plasma renin activity and plasma aldosterone induced by its mild antimineralocorticoid activity.
Use in Specific Populations
Pregnancy
- There is little or no increased risk of birth defects in women who inadvertently use COCs during early pregnancy. Epidemiologic studies and meta-analyses have not found an increased risk of genital or non-genital birth defects (including cardiac anomalies and limb-reduction defects) following exposure to low dose COCs prior to conception or during early pregnancy.
- The administration of COCs to induce withdrawal bleeding should not be used as a test for pregnancy. COCs should not be used during pregnancy to treat threatened or habitual abortion.
- Women who do not breastfeed may start COCs no earlier than four weeks postpartum.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Drospirenone and Ethinyl estradiol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Drospirenone and Ethinyl estradiol during labor and delivery.
Nursing Mothers
- When possible, advise the nursing mother to use other forms of contraception until she has weaned her child. Estrogen-containing COCs can reduce milk production in breastfeeding mothers. This is less likely to occur once breastfeeding is well-established; however, it can occur at any time in some women. Small amounts of oral contraceptive steroids and/or metabolites are present in breast milk.
- After oral administration of 3 mg DRSP/0.03 mg EE (Yasmin) tablets, about 0.02% of the DRSP dose was excreted into the breast milk of postpartum women within 24 hours. This results in a maximal daily dose of about 0.003 mg DRSP in an infant.
Pediatric Use
- Safety and efficacy of Nikki have been established in women of reproductive age. Efficacy are expected to be the same for postpubertal adolescents under the age of 18 and for users 18 years and older. Use of this product before menarche is not indicated.
Geriatic Use
- Nikki has not been studied in postmenopausal women and is not indicated in this population.
Gender
There is no FDA guidance on the use of Drospirenone and Ethinyl estradiol with respect to specific gender populations.
Race
- No clinically significant difference was observed between the pharmacokinetics of DRSP or EE in Japanese versus Caucasian women.
Renal Impairment
- Nikki is contraindicated in patients with renal impairment.
- In subjects with creatinine clearance (CLcr) of 50-79 mL/min, serum DRSP levels were comparable to those in a control group with CLcr ≥80 mL/min. *In subjects with serum DRSP concentrations were on average 37% higher than those in the control group. In addition, there is a potential to develop hyperkalemia in subjects with renal impairment whose serum potassium is in the upper reference range, and who are concomitantly using potassium sparing drugs.
Hepatic Impairment
- Nikki is contraindicated in patients with hepatic disease. The mean exposure to DRSP in women with moderate liver impairment is approximately three times higher than the exposure in women with normal liver function. Nikki has not been studied in women with severe hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Drospirenone and Ethinyl estradiol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Drospirenone and Ethinyl estradiol in patients who are immunocompromised.
Administration and Monitoring
Administration
How to Take Nikki
- Take one tablet by mouth at the same time every day. The failure rate may increase when pills are missed or taken incorrectly.
- To achieve maximum contraceptive effectiveness, Nikki must be taken exactly as directed, in the order directed on the wallet. Single missed pills should be taken as soon as remembered.
How to Start Nikki
- Instruct the patient to begin taking Nikki either on the first day of her menstrual period (Day 1 Start) or on the first Sunday after the onset of her menstrual period (Sunday Start).
Day 1 Start
- During the first cycle of Nikki use, instruct the patient to take one pink Nikki daily, beginning on Day 1 of her menstrual cycle. (The first day of menstruation is Day 1.) She should take one pink Nikki daily for 24 consecutive days, followed by one white inert tablet daily on Days 25 through 28. Nikki should be taken in the order directed on the package at the same time each day, preferably after the evening meal or at bedtime with some liquid, as needed. Nikki can be taken without regard to meals. If Nikki is first taken later than the first day of the menstrual cycle, Nikki should not be considered effective as a contraceptive until after the first 7 consecutive days of product administration. Instruct the patient to use a non-hormonal contraceptive as back-up during the first 7 days. The possibility of ovulation and conception prior to initiation of medication should be considered.
Sunday Start
- During the first cycle of Nikki use, instruct the patient to take one pink Nikki daily, beginning on the first Sunday after the onset of her menstrual period. She should take one pink Nikki daily for 24 consecutive days, followed by one white inert tablet daily on Days 25 through 28. Nikki should be taken in the order directed on the package at the same time each day, preferably after the evening meal or at bedtime with some liquid, as needed. Nikki can be taken without regard to meals. Nikki should not be considered effective as a contraceptive until after the first 7 consecutive days of product administration. Instruct the patient to use a non-hormonal contraceptive as back-up during the first 7 days. The possibility of ovulation and conception prior to initiation of medication should be considered.
- The patient should begin her next and all subsequent 28-day regimens of Nikki on the same day of the week that she began her first regimen, following the same schedule. She should begin taking her pink tablets on the next day after ingestion of the last white tablet, regardless of whether or not a menstrual period has occurred or is still in progress. Anytime a subsequent cycle of Nikki is started later than the day following administration of the last white tablet, the patient should use another method of contraception until she has taken a pink Nikki daily for seven consecutive days.
When switching from a different birth control pill
- When switching from another birth control pill, Nikki should be started on the same day that a new pack of the previous oral contraceptive would have been started.
When switching from a method other than a birth control pill
- When switching from a transdermal patch or vaginal ring, Nikki should be started when the next application would have been due. When switching from an injection, Nikki should be started when the next dose would have been due. When switching from an intrauterine contraceptive or an implant, Nikki should be started on the day of removal.
- Withdrawal bleeding usually occurs within 3 days following the last pink tablet. If spotting or breakthrough bleeding occurs while taking Nikki, instruct the patient to continue taking Nikki by the regimen described above. Counsel her that this type of bleeding is usually transient and without significance; however, advise her that if the bleeding is persistent or prolonged, she should consult her healthcare provider.
- Although the occurrence of pregnancy is low if Nikki is taken according to directions, if withdrawal bleeding does not occur, consider the possibility of pregnancy. If the patient has not adhered to the prescribed dosing schedule (missed one or more active tablets or started taking them on a day later than she should have), consider the possibility of pregnancy at the time of the first missed period and take appropriate diagnostic measures. If the patient has adhered to the prescribed regimen and misses two consecutive periods, rule out pregnancy. Discontinue Nikki if pregnancy is confirmed.
- The risk of pregnancy increases with each active pink tablet missed. For additional patient instructions regarding missed pills, see the "What to Do if You Miss Pills" section in the FDA Approved Patient Labeling. If breakthrough bleeding occurs following missed tablets, it will usually be transient and of no consequence. If the patient misses one or more white tablets, she should still be protected against pregnancy provided she begins taking a new cycle of pink tablets on the proper day.
- For postpartum women who do not breastfeed or after a second trimester abortion, start Nikki no earlier than 4 weeks postpartum due to the increased risk of thromboembolism. If the patient starts on Nikki postpartum and has not yet had a period, evaluate for possible pregnancy, and instruct her to use an additional method of contraception until she has taken Nikki for 7 consecutive days.
Advice in Case of Gastrointestinal Disturbances
- In case of severe vomiting or diarrhea, absorption may not be complete and additional contraceptive measures should be taken. If vomiting occurs within 3 to 4 hours after tablet-taking, this can be regarded as a missed tablet.
Close
DOSAGE FORMS AND STRENGTHS
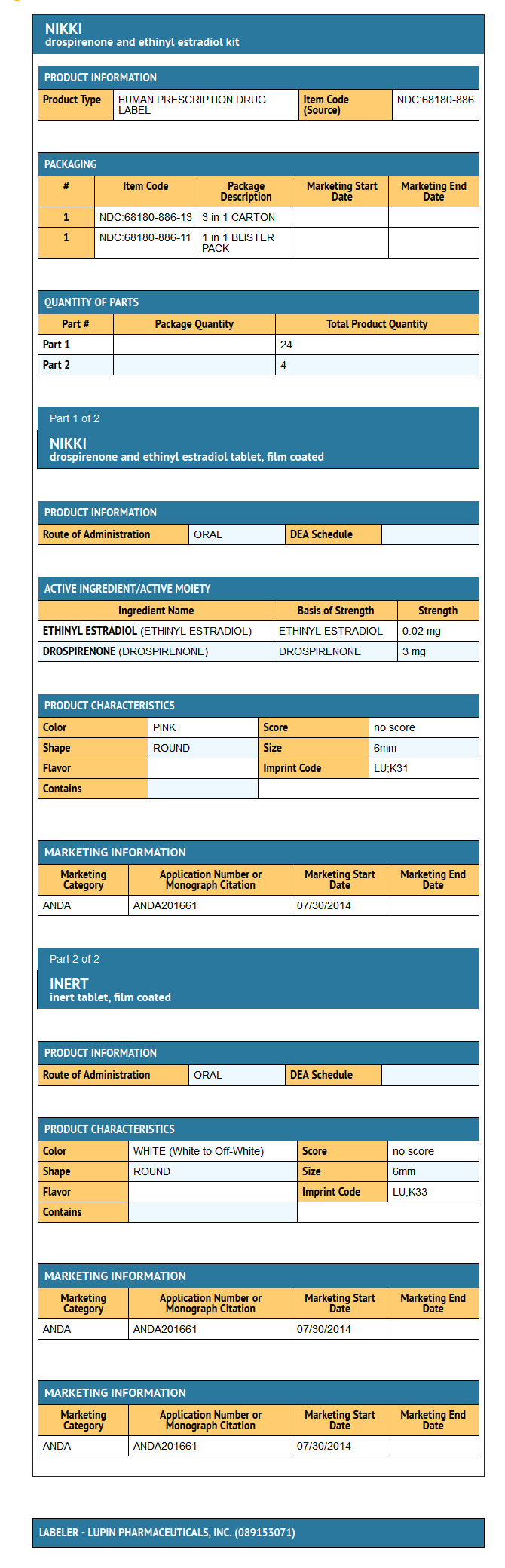
- Nikki (drospirenone and ethinyl estradiol tablets USP), 3 mg/0.02 mg are available in wallet packs.
- Each wallet pack (28 round, biconvex film-coated tablets) contains in the following order:
- 24 pink tablets each containing 3 mg drospirenone (DRSP) and 0.02 mg ethinyl estradiol (EE)
- 4 white to off-white inert tablets
Monitoring
- Carbohydrate and lipid metabolic effects : Monitor prediabetic and diabetic women taking Nikki. COCs may decrease glucose intolerance in a dose-related fashion. Consider an alternate contraceptive method for women with uncontrolled dyslipidemia.
- For women with well-controlled hypertension, monitor blood pressure and stop Nikki if blood pressure rises significantly. Women with uncontrolled hypertension or hypertension with vascular disease should not use COCs.
IV Compatibility
There is limited information regarding the compatibility of Drospirenone and Ethinyl estradiol and IV administrations.
Overdosage
- There have been no reports of serious ill effects from overdose, including ingestion by children. Overdosage may cause withdrawal bleeding in females and nausea.
- DRSP is a spironolactone analogue which has antimineralocorticoid properties. Serum concentration of potassium and sodium, and evidence of metabolic acidosis, should be monitored in cases of overdose.
Pharmacology
There is limited information regarding Drospirenone and Ethinyl estradiol Pharmacology in the drug label.
Mechanism of Action
- COCs lower the risk of becoming pregnant primarily by suppressing ovulation. Other possible mechanisms may include cervical mucus changes that inhibit sperm penetration and the endometrial changes that reduce the likelihood of implantation.
Structure
- Nikki (drospirenone and ethinyl estradiol tablets USP), 3 mg/0.02 mg provides an oral contraceptive regimen consisting of 24 pink, round, biconvex active film-coated tablets each containing 3 mg of drospirenone and 0.02 mg of ethinyl estradiol and 4 white to off-white inert film-coated tablets.
- The inactive ingredients in the pink film-coated tablets are corn starch, hypromellose, iron oxide red, lactose monohydrate, magnesium stearate, pregelatinised starch, talc and titanium dioxide. The white to off-white inert film-coated tablets contain corn starch, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, pregelatinized starch and titanium dioxide.
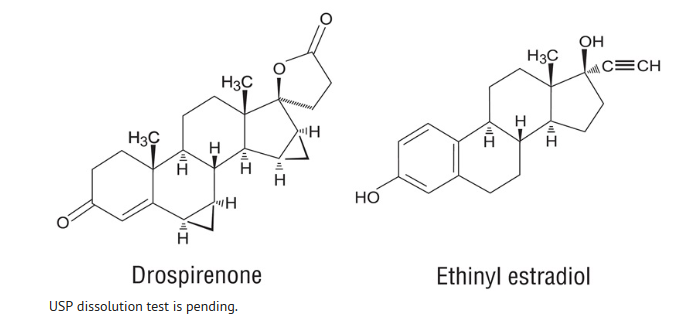
- Drospirenone (6R, 7R, 8R, 9S, 10R, 13S, 14S, 15S, 16S, 17S) - 1, 3', 4', 6, 6a, 7, 8, 9, 10, 11, 12,13,14,15,15a,16-hexadecahydro-10,13-dimethylspiro-[17H-dicyclopropa- [6,7:15,16] cyclopenta [a] phenanthrene- 17, 2' (5H)- furan]-3, 5'(2H)-dione) is a synthetic progestational compound and has a molecular weight of 366.5 and a molecular formula of C24H30O3.
- Ethinyl estradiol (19-nor-17a-pregna 1, 3, 5(10)-triene-20-yne-3, 17-diol) is a synthetic estrogenic compound and has a molecular weight of 296.4 and a molecular formula of C20H24O2.
- The structural formulas are as follows:
Pharmacodynamics
- Drospirenone is a spironolactone analogue with antimineralocorticoid and antiandrogenic activity. The estrogen in Nikki is ethinyl estradiol.
Contraception
- Two studies evaluated the effect of 3 mg DRSP / 0.02 mg EE combinations on the suppression of ovarian activity as assessed by measurement of follicle size via transvaginal ultrasound and serum hormone (progesterone and estradiol) analyses during two treatment cycles (21-day active tablet period plus 7-day pill-free period). More than 90% of subjects in these studies demonstrated ovulation inhibition. One study compared the effect of 3 mg DRSP/0.02 mg EE combinations with two different regimens (24-day active tablet period plus 4-day pill-free period vs. 21-day active tablet period plus 7-day pill-free period) on the suppression of ovarian activity during two treatment cycles. During the first treatment cycle, there were no subjects (0/49, 0%) taking the 24-day regimen who ovulated compared to 1 subject (1/50, 2%) using the 21-day regimen. After intentionally introduced dosing errors (3 missed active tablets on Days 1 to 3) during the second treatment cycle, there was 1 subject (1/49, 2%) taking the 24-day regimen who ovulated compared to 4 subjects (4/50, 8%) using the 21-day regimen.
Acne
- Acne vulgaris is a skin condition with a multifactorial etiology including androgen stimulation of sebum production. While the combination of EE and DRSP increases sex hormone binding globulin (SHBG) and decreases free testosterone, the relationship between these changes and a decrease in the severity of facial acne in otherwise healthy women with this skin condition has not been established. The impact of the antiandrogenic activity of DRSP on acne is not known.
Pharmacokinetics
Absorption
- The absolute bioavailability of DRSP from a single entity tablet is about 76%. The absolute bioavailability of EE is approximately 40% as a result of presystemic conjugation and first-pass metabolism. The absolute bioavailability of Nikki, which is a combination tablet of DRSP and EE, has not been evaluated. Serum concentrations of DRSP and EE reached peak levels within 1-2 hours after administration of Nikki.
- The pharmacokinetics of DRSP are dose proportional following single doses ranging from 1-10 mg. Following daily dosing of Nikki, steady state DRSP concentrations were observed after 8 days. There was about 2 to 3 fold accumulation in serum Cmax and AUC (0-24h) values of DRSP following multiple dose administration of Nikki (see Table 2).
- For EE, steady-state conditions are reported during the second half of a treatment cycle. Following daily administration of Nikki, serum Cmax and AUC (0-24h) values of EE accumulate by a factor of about 1.5 to 2 (see Table 2).
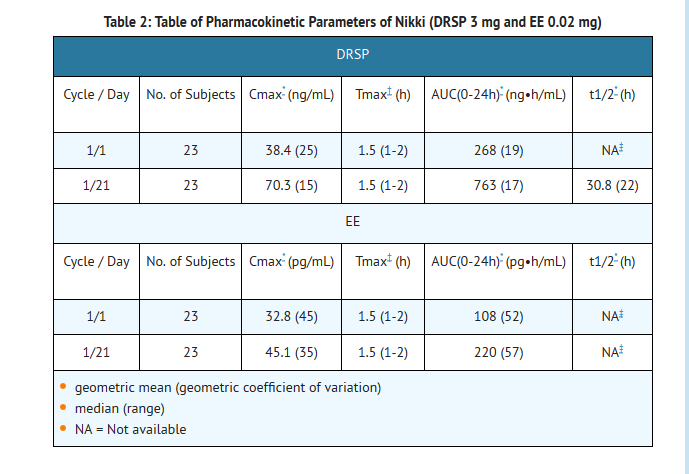
- Food Effect:
- The rate of absorption of DRSP and EE following single administration of a formulation similar to Nikki was slower under fed (high fat meal) conditions with the serum Cmax being reduced about 40% for both components. The extent of absorption of DRSP, however, remained unchanged. In contrast, the extent of absorption of EE was reduced by about 20% under fed conditions.
Distribution
- DRSP and EE serum concentrations decline in two phases. The apparent volume of distribution of DRSP is approximately 4 L/kg and that of EE is reported to be approximately 4-5 L/kg.
- DRSP does not bind to SHBG or corticosteroid binding globulin (CBG) but binds about 97% to other serum proteins. Multiple dosing over 3 cycles resulted in no change in the free fraction (as measured at trough concentrations). EE is reported to be highly but non-specifically bound to serum albumin (approximately 98.5%) and induces an increase in the serum concentrations of both SHBG and CBG. EE induced effects on SHBG and CBG were not affected by variation of the DRSP dosage in the range of 2 to 3 mg.
Metabolism
- The two main metabolites of DRSP found in human plasma were identified to be the acid form of DRSP generated by opening of the lactone ring and the 4, 5-dihydrodrospirenone-3-sulfate. These metabolites were shown not to be pharmacologically active. In in vitro studies with human liver microsomes, DRSP was metabolized only to a minor extent mainly by CYP3A4.
- EE has been reported to be subject to presystemic conjugation in both small bowel mucosa and the liver. Metabolism occurs primarily by aromatic hydroxylation but a wide variety of hydroxylated and methylated metabolites are formed. These are present as free metabolites and as conjugates with glucuronide and sulfate. CYP3A4 in the liver is responsible for the 2-hydroxylation which is the major oxidative reaction. The 2-hydroxy metabolite is further transformed by methylation and glucuronidation prior to urinary and fecal excretion.
Excretion
- DRSP serum concentrations are characterized by a terminal disposition phase half-life of approximately 30 hours after both single and multiple dose regimens. Excretion of DRSP was nearly complete after ten days and amounts excreted were slightly higher in feces compared to urine. DRSP was extensively metabolized and only trace amounts of unchanged DRSP were excreted in urine and feces. At least 20 different metabolites were observed in urine and feces. About 38-47% of the metabolites in urine were glucuronide and sulfate conjugates. In feces, about 17-20% of the metabolites were excreted as glucuronides and sulfates.
- For EE the terminal disposition phase half-life has been reported to be approximately 24 hours. EE is not excreted unchanged. EE is excreted in the urine and feces as glucuronide and sulfate conjugates and undergoes enterohepatic circulation.
Use in Specific Populations
- Pediatric Use:
- Safety and efficacy of Nikki has been established in women of reproductive age. Efficacy is expected to be the same for postpubertal adolescents under the age of 18 and for users 18 years and older. Use of this product before menarche is not indicated.
- Geriatric Use:
- Nikki has not been studied in postmenopausal women and is not indicated in this population.
- Race:
- No clinically significant difference was observed between the pharmacokinetics of DRSP or EE in Japanese versus Caucasian women (age 25-35) when 3 mg DRSP/0.02 mg EE was administered daily for 21 days. Other ethnic groups have not been specifically studied.
- Renal Impairment:
- Nikki is contraindicated in patients with renal impairment.
- The effect of renal impairment on the pharmacokinetics of DRSP (3 mg daily for 14 days) and the effect of DRSP on serum potassium concentrations were investigated in three separate groups of female subjects (n=28, age 30–65). All subjects were on a low potassium diet. During the study, 7 subjects continued the use of potassium-sparing drugs for the treatment of their underlying illness. On the 14th day (steady-state) of DRSP treatment, the serum DRSP concentrations in the group with CLcr of 50–79 mL/min were comparable to those in the control group with CLcr ≥ 80 mL/min. The serum DRSP concentrations were on average 37% higher in the group with CLcr of 30–49 mL/min compared to those in the control group. DRSP treatment did not show any clinically significant effect on serum potassium concentration. Although hyperkalemia was not observed in the study, in five of the seven subjects who continued use of potassium-sparing drugs during the study, mean serum potassium concentrations increased by up to 0.33 mEq/L.
- Hepatic Impairment:
- Nikki is contraindicated in patients with hepatic disease.
- The mean exposure to DRSP in women with moderate liver impairment is approximately three times higher than the exposure in women with normal liver function. Nikki has not been studied in women with severe hepatic impairment.
Drug Interactions
- Consult the labeling of all concurrently used drugs to obtain further information about interactions with oral contraceptives or the potential for enzyme alterations.
- Effects of Other Drugs on Combined Oral Contraceptives:
- Substances Diminishing the Efficacy of CoCs: Drugs or herbal products that induce certain enzymes, including CYP3A4, may decrease the effectiveness of COCs or increase breakthrough bleeding.
- Substances Increasing the Plasma Concentrations of COCs: Co-administration of atorvastatin and certain COCs containing ethinyl estradiol increase AUC values for ethinyl estradiol by approximately 20%. Ascorbic acid and acetaminophen may increase plasma ethinyl estradiol concentrations, possibly by inhibition of conjugation. CYP3A4 inhibitors such as itraconazole or ketoconazole may increase plasma hormone concentrations.
- HIV/HCV Protease Inhibitors and Non-nucleoside Reverse Transcriptase Inhibitors: Significant changes (increase or decrease) in the plasma concentrations of estrogen and progestin have been noted in some cases of co-administration with HIV/HCV protease inhibitors or with non-nucleoside reverse transcriptase inhibitors.
- Antibiotics: There have been reports of pregnancy while taking hormonal contraceptives and antibiotics, but clinical pharmacokinetic studies have not shown consistent effects of antibiotics on plasma concentrations of synthetic steroids.
Effects of Combined Oral Contraceptives on Other Drugs:
- COCs containing ethinyl estradiol may inhibit the metabolism of other compounds. COCs have been shown to significantly decrease plasma concentrations of lamotrigine, likely due to induction of lamotrigine glucuronidation. This may reduce seizure control; therefore, dosage adjustments of lamotrigine may be necessary. Consult the labeling of the concurrently-used drug to obtain further information about interactions with COCs or the potential for enzyme alterations.
- Metabolism of DRSP and potential effects of DRSP on hepatic CYP enzymes have been investigated in in vitro and in vivo studies. In in vitro studies DRSP did not affect turnover of model substrates of CYP1A2 and CYP2D6, but had an inhibitory influence on the turnover of model substrates of CYP1A1, CYP2C9, CYP2C19 and CYP3A4, with CYP2C19 being the most sensitive enzyme. The potential effect of DRSP on CYP2C19 activity was investigated in a clinical pharmacokinetic study using omeprazole as a marker substrate. In the study with 24 postmenopausal women [including 12 women with homozygous (wild type) CYP2C19 genotype and 12 women with heterozygous CYP2C19 genotype] the daily oral administration of 3 mg DRSP for 14 days did not affect the oral clearance of omeprazole (40 mg, single oral dose) and the CYP2C19 product 5-hydroxy omeprazole. Furthermore, no significant effect of DRSP on the systemic clearance of the CYP3A4 product omeprazole sulfone was found. These results demonstrate that DRSP did not inhibit CYP2C19 and CYP3A4 in vivo.
- Two additional clinical drug-drug interaction studies using simvastatin and midazolam as marker substrates for CYP3A4 were each performed in 24 healthy postmenopausal women. The results of these studies demonstrated that pharmacokinetics of the CYP3A4 substrates were not influenced by steady state DRSP concentrations achieved after administration of 3 mg DRSP/day.
- Women on thyroid hormone replacement therapy may need increased doses of thyroid hormone because serum concentration of thyroid-binding globulin increases with use of COCs.
Interactions With Drugs That Have the Potential to Increase Serum Potassium Concentration:
- There is a potential for an increase in serum potassium concentration in women taking Nikki with other drugs that may increase serum potassium concentration.
- A drug-drug interaction study of DRSP 3 mg/estradiol (E2) 1 mg versus placebo was performed in 24 mildly hypertensive postmenopausal women taking enalapril maleate 10 mg twice daily. Potassium concentrations were obtained every other day for a total of 2 weeks in all subjects. Mean serum potassium concentrations in the DRSP/E2 treatment group relative to baseline were 0.22 mEq/L higher than those in the placebo group. Serum potassium concentrations also were measured at multiple time points over 24 hours at baseline and on Day 14. On Day 14, the ratios for serum potassium Cmax and AUC in the DRSP/E2 group to those in the placebo group were 0.955 (90% CI: 0.914, 0.999) and 1.010 (90% CI: 0.944, 1.08), respectively. No patient in either treatment group developed hyperkalemia (serum potassium concentrations > 5.5 mEq/L).
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- In a 24 month oral carcinogenicity study in mice dosed with 10 mg/kg/day DRSP alone or 1 + 0.01, 3 + 0.03 and 10 + 0.1 mg/kg/day of DRSP and EE, 0.1 to 2 times the exposure (AUC of DRSP) of women taking a contraceptive dose, there was an increase in carcinomas of the harderian gland in the group that received the high dose of DRSP alone. In a similar study in rats given 10 mg/kg/day DRSP alone or 0.3 + 0.003, 3 + 0.03 and 10 + 0.1 mg/kg/day DRSP and EE, 0.8 to 10 times the exposure of women taking a contraceptive dose, there was an increased incidence of benign and total (benign and malignant) adrenal gland pheochromocytomas in the group receiving the high dose of DRSP. Mutagenesis studies for DRSP were conducted in vivo and in vitro and no evidence of mutagenic activity was observed.
Clinical Studies
Oral Contraceptive Clinical Trial
- In the primary contraceptive efficacy study of Nikki (3 mg DRSP/0.02 mg EE) of up to 1 year duration, 1,027 subjects were enrolled and completed 11,480 28-day cycles of use. The age range was 17 to 36 years. The racial demographic was: 87.8% Caucasian, 4.6% Hispanic, 4.3% Black, 1.2% Asian, and 2.1% other. Women with a BMI greater than 35 were excluded from the trial. The pregnancy rate (Pearl Index) was 1.41 (95% CI [0.73, 2.47]) per 100 woman-years of use based on 12 pregnancies that occurred after the onset of treatment and within 14 days after the last dose of Nikki in women 35 years of age or younger during cycles in which no other form of contraception was used.
Acne Clinical Trials
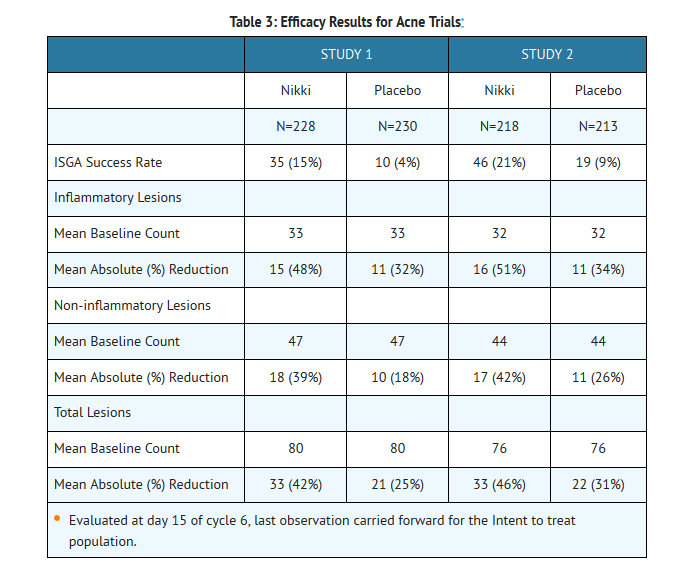
- In two multicenter, double-blind, randomized, placebo-controlled studies, 889 subjects, ages 14 to 45 years, with moderate acne received Nikki or placebo for six 28-day cycles. The primary efficacy endpoints were the percent change in inflammatory lesions, non-inflammatory lesions, total lesions, and the percentage of subjects with a "clear" or "almost clear" rating on the Investigator's Static Global Assessment (ISGA) scale on day 15 of cycle 6, as presented in Table 3:
How Supplied
- Nikki (drospirenone and ethinyl estradiol tablets USP), 3 mg/0.02 mg is available in a wallet (NDC 68180-886-11) packed in a pouch (NDC 68180-886-11). Such three pouches are packaged in a carton (NDC 68180-886-13).
- Each wallet pack (28 film-coated tablets) contains in the following order:
- 24 active pink, round, biconvex, film-coated tablets, debossed with "LU" on one side and "K31" on the other side each containing 3 mg drospirenone and 0.02 mg ethinyl Estradiol
- 4 inert white to off-white round, biconvex film-coated tablets debossed with "K33" on one side and "LU" on the other side.
Storage
- Store at 25°C (77°F); excursions permitted to 15 to 30°C (59 to 86°F) [See USP Controlled Room Temperature].
Images
Drug Images
{{#ask: Page Name::Drospirenone and Ethinyl estradiol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Drospirenone and Ethinyl estradiol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- See FDA-approved patient labeling (Patient Information)
- Counsel patients that cigarette smoking increases the risk of serious cardiovascular events from COC use, and that woman who are over 35 years old and smoke should not use COCs.
- Counsel patients that the increased risk of VTE compared to non-users of COCs is greatest after initially starting a COC or restarting (following a 4 week or greater pill-free interval) the same or a different COC.
- Counsel patients about the information regarding the risk of VTE with DRSP-containing COCs compared to COCs that contain levonorgestrel or some other progestins.
- Counsel patients that Nikki does not protect against HIV-infection (AIDS) and other sexually transmitted diseases.
- Counsel patients on Warnings and Precautions associated with COCs.
- Counsel patients that Nikki contains DRSP. Drospirenone may increase potassium. Patients should be advised to inform their healthcare provider if they have kidney, liver or adrenal disease because the use of Nikki in the presence of these conditions could cause serious heart and health problems. They should also inform their healthcare provider if they are currently on daily, long-term treatment (NSAIDs, potassium-sparing diuretics, potassium supplementation, ACE inhibitors, angiotensin-II receptor antagonists, heparin or aldosterone antagonists) for a chronic condition.
- Inform patients that Nikki is not indicated during pregnancy. If pregnancy occurs during treatment with Nikki, instruct the patient to stop further intake.
- Counsel patients to take one tablet daily by mouth at the same time every day. Instruct patients what to do in the event pills are missed. See "WHAT to DO if YOU MISS PILLS " section in FDA-Approved Patient Labeling .
- Counsel patients to use a back-up or alternative method of contraception when enzyme inducers are used with COCs.
- Counsel patients who are breastfeeding or who desire to breastfeed that COCs may reduce breast milk production. This is less likely to occur if breastfeeding is well established.
- Counsel any patient who starts COCs postpartum, and who have not yet had a period, to use an additional method of contraception until she has taken a pink tablet for 7 consecutive days.
- Counsel patients that amenorrhea may occur. Rule out pregnancy in the event of amenorrhea in two or more consecutive cycles.

Precautions with Alcohol
- Alcohol-Drospirenone and Ethinyl estradiol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Yasmin YAZ Yaz 28 Ocella Gianvi Zarah Syeda Loryna
Look-Alike Drug Names
There is limited information regarding Drospirenone and Ethinyl estradiol Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.