Cytarabine liposome
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
CHEMICAL ARACHNOIDITIS
|
Overview
Cytarabine liposome is an antimetabolite that is FDA approved for the treatment of lymphomatous meningitis. There is a Black Box Warning for this drug as shown here. Common adverse reactions include headache , nausea, vomiting , arachnoiditis, weakness, confusion, pyrexia, fatigue, constipation, back pain, gait abnormal , convulsions , dizziness , lethargy, insomnia, urinary tract infection , neck pain, dehydration, anemia , diarrhea , appetite decreased , thrombocytopenia, edema peripheral, arthralgia, neck stiffness vision blurred, muscle weakness, neutropenia, hypoesthesia, agitation, and dyspnea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Cytarabine liposome® (cytarabine liposome injection) is indicated for the intrathecal treatment of lymphomatous meningitis.
Preparation and Administration Precautions
- Cytarabine liposome is a cytotoxic anticancer drug and, as with other potentially toxic compounds, caution should be used in handling Cytarabine liposome. The use of gloves is recommended. If Cytarabine liposome suspension contacts the skin, wash immediately with soap and water. If it contacts mucous membranes, flush thoroughly with water.
Preparation and Administration
- No further reconstitution or dilution is required. Cytarabine liposome particles have a tendency to settle with time. Vials of Cytarabine liposome should be allowed to warm to room temperature and gently agitated or inverted to re-suspend the particles immediately prior to withdrawal from the vial. Avoid aggressive agitation.
- Cytarabine liposome should be withdrawn from the vial immediately before administration. Cytarabine liposome is a single dose vial and does not contain any preservative. Cytarabine liposome should be used within 4 hours of withdrawal from the vial. Unused portions of each vial should be discarded properly . Do not save any unused portions for later administration. Do not mix Cytarabine liposome with any other medications.
Dosing Precautions
- In-line filters must not be used when administering Cytarabine liposome. Cytarabine liposome is administered directly into the cerebrospinal fluid (CSF) via an intraventricular reservoir or by direct injection into the lumbar sac. Cytarabine liposome should be injected slowly over a period of 1-5 minutes. Following drug administration by lumbar puncture, the patient should be instructed to lie flat for 1 hour. Patients should be observed by the physician for immediate toxic reactions.
Dosing Regimen
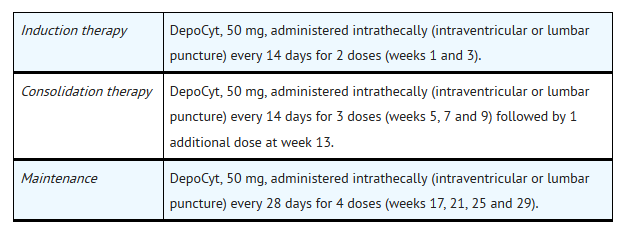
- For the treatment of lymphomatous meningitis, Cytarabine liposome 50 mg (one vial of Cytarabine liposome) is recommended to be given according to the following schedule:
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cytarabine liposome in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cytarabine liposome in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Cytarabine liposome in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cytarabine liposome in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cytarabine liposome in pediatric patients.
Contraindications
- Cytarabine liposome® (cytarabine liposome injection) is contraindicated in patients who are hypersensitive to cytarabine or any component of the formulation, and in patients with active meningeal infection.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
CHEMICAL ARACHNOIDITIS
|
Chemical Arachnoiditis
- chemical arachnoiditis, a syndrome manifested primarily by nausea, vomiting, headache and fever, has been a common adverse event in all studies. If chemical arachnoiditis is suspected, exclude other inflammatory, infectious, or neoplastic conditions. If left untreated, chemical arachnoiditis may be fatal. The incidence and severity of chemical arachnoiditis can be reduced by coadministration of dexamethasone. Patients receiving Cytarabine liposome should be treated concurrently with dexamethasone to mitigate the symptoms of chemical arachnoiditis .
- Toxic effects may be related to a single dose or to cumulative administration. Because toxic effects can occur at any time during therapy (although they are most likely to occur within 5 days of drug administration), patients receiving intrathecal therapy with Cytarabine liposome should be monitored continuously for the development of neurotoxicity. If patients develop neurotoxicity, reduce subsequent doses of Cytarabine liposome. If neurotoxicity persists, discontinue Cytarabine liposome.
- Hydrocephalus has also been reported, possibly precipitated by arachnoiditis.
- Arachnoiditis is an expected and well-documented side effect of both neoplastic meningitis and of . The incidence of severe and life-threatening arachnoiditis in patients receiving Cytarabine liposome was 19% (48/257) in all patients and 30% (10/33) in patients with lymphomatous meningitis. In the early dose-finding study, chemical arachnoiditis was observed in 100% of cycles without dexamethasone prophylaxis. When concurrent dexamethasone was administered, chemical arachnoiditis was observed in 33% of cycles.
Neurotoxicity
- Intrathecal administration of cytarabine may cause myelopathy and other neurologic toxicity and can rarely lead to a permanent neurologic deficit. Administration of intrathecal cytarabine in combination with other chemotherapeutic agents or with cranial/spinal irradiation may increase this risk of neurotoxicity.
- Blockage to CSF flow may result in increased free cytarabine concentrations in the CSF and an increased risk of neurotoxicity. Therefore, as with any intrathecal cytotoxic therapy, consideration should be given to the need for assessment of CSF flow before treatment is started.
- Following intrathecal administration of Cytarabine liposome, central nervous system toxicity, including persistent extreme somnolence, hemiplegia, visual disturbances including blindness which may be total and permanent, deafness and cranial nerve palsies have been reported. Symptoms and signs of peripheral neuropathy, such as pain, numbness, paresthesia, weakness, and impaired bowel and bladder control have also been observed. In some cases, a combination of neurological signs and symptoms has been reported as Cauda Equina Syndrome.
- If patients develop neurotoxicity, reduce subsequent doses of Cytarabine liposome or discontinue Cytarabine liposome. Headache, nausea, and fever are expected in early signs of neurotoxicity.
Transient Elevations in CSF Protein and CSF White Blood Cells
- Transient elevations in CSF protein and white blood cell counts have been observed in patients following Cytarabine liposome administration.
Embryo-fetal Toxicity
- Cytarabine, the active component of Cytarabine liposome, can cause fetal harm if a pregnant woman is exposed to the drug systemically. The systemic exposure of cytarabine following intrathecal administration of Cytarabine liposome is negligible. Cytarabine was teratogenic in mice and rats. Cytarabine was embryotoxic in mice when administered during the period of organogenesis. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential harm to a fetus.
Adverse Reactions
Clinical Trials Experience
- The following serious adverse reactions are described in greater detail in other sections of the label:
- Chemical Arachnoiditis
- Neurotoxicity
- Transient elevations in CSF protein and CSF white blood cells
Most Frequently Reported Reactions
- After intrathecal administration of cytarabine the most frequently reported reactions (≥ 10%) are headache NOS, nausea, vomiting NOS, arachnoiditis, weakness, confusion, pyrexia, fatigue, constipation, back pain, gait abnormal NOS, convulsions NOS, dizziness NOS, lethargy, pain in limb, insomnia, urinary tract infection NOS, neck pain, death NOS, pain, memory impairment, dehydration, anemia NOS, diarrhea NOS, appetite decreased NOS, thrombocytopenia, edema peripheral, arthralgia, neck stiffness, vision blurred, muscle weakness, neutropenia, hypoesthesia, agitation, and dyspnea NOS.
Clinical Trials
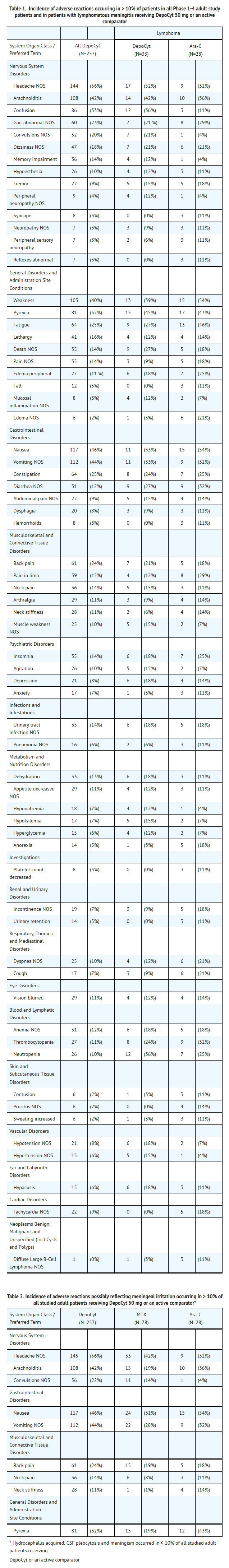
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The toxicity database consists of the observations made during Phase 1-4 studies. The most common adverse reactions in all patients and in patients with lymphoma are shown in TABLE 1. The incidences of symptoms possibly reflecting meningeal irritation are shown in TABLE 2.
- During the clinical studies, 2 deaths related to Cytarabine liposome were reported. One patient at the 125 mg dose level died of encephalopathy 36 hours after receiving an intraventricular dose of Cytarabine liposome. This patient, however, was also receiving concomitant whole brain irradiation and had previously received intraventricular methotrexate. The other patient received Cytarabine liposome, 50 mg by the intraventricular route and developed focal seizures progressing to status epilepticus. This patient died approximately 8 weeks after the last dose of study medication. In the controlled lymphoma study, the patient incidence of seizures was higher in the Cytarabine liposome group (4/17, 23.5%) than in the cytarabine group (1/16, 6.3%). The death of 1 additional patient was considered “possibly” related to Cytarabine liposome. He was a 63-year-old with extensive lymphoma involving the nasopharynx, brain, and meninges with multiple neurologic deficits who died of apparent disease progression 4 days after his second dose of Cytarabine liposome.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Cytarabine liposome in the drug label.
Drug Interactions
- No formal assessments of pharmacokinetic drug-drug interactions between Cytarabine liposome and other agents have been conducted. Concomitant administration of Cytarabine liposome with other antineoplastic agents administered by the intrathecal route has not been studied. With intrathecal cytarabine and other cytotoxic agents administered intrathecally, enhanced neurotoxicity has been associated with coadministration of drugs.
Use in Specific Populations
Pregnancy
Risk Summary
- There are no studies assessing the reproductive toxicity of Cytarabine liposome. The systemic exposure of cytarabine following intrathecal administration of Cytarabine liposome is negligible. Cytarabine can cause fetal harm if a pregnant woman is exposed to the drug systemically. Three anecdotal cases of major limb malformations have been reported in infants after their mothers received intravenous cytarabine, alone or in combination with other agents, during the first trimester. Advise women of childbearing potential to avoid becoming pregnant while receiving Cytarabine liposome. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential harm to a fetus.
Animal Data
- Cytarabine was teratogenic in mice (cleft palate, phocomelia, deformed appendages, skeletal abnormalities) when doses ≥2 mg/kg/day were administered IP during the period of organogenesis (about 0.2 times the recommended human dose on a mg/m2 basis), and in rats (deformed appendages) when 20 mg/kg was administered as a single IP dose on day 12 of gestation (about 4 times the recommended human dose on a mg/m2 basis). Single IP doses of 50 mg/kg in rats (about 10 times the recommended human dose on a mg/m2 basis) on day 14 of gestation reduced prenatal and postnatal brain size and permanent impairment of learning ability.
- Cytarabine was embryotoxic in mice when administered during the period of organogenesis. Embryotoxicity was characterized by decreased fetal weight at 0.5 mg/kg/day (about 0.05 times the recommended human dose on mg/m2 basis), and increased early and late resorptions and decreased live litter sizes at 8 mg/kg/day (approximately equal to the recommended human dose on mg/m2basis).
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cytarabine liposome in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Cytarabine liposome during labor and delivery.
Nursing Mothers
- It is not known whether cytarabine is excreted in human milk following intrathecal Cytarabine liposome administration. The systemic exposure to free cytarabine following intrathecal treatment with Cytarabine liposome was negligible. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
- The safety and efficacy of Cytarabine liposome in pediatric patients has not been established.
Geriatic Use
There is no FDA guidance on the use of Cytarabine liposome with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Cytarabine liposome with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cytarabine liposome with respect to specific racial populations.
Renal Impairment
- The effects of hepatic or renal impairment on the pharmacokinetics of Cytarabine liposome have not been studied.
Hepatic Impairment
- The effects of hepatic or renal impairment on the pharmacokinetics of Cytarabine liposome have not been studied.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Cytarabine liposome in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Cytarabine liposome in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intrathecal
Monitoring
There is limited information regarding Monitoring of Cytarabine liposome in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Cytarabine liposome in the drug label.
Overdosage
- No overdosages with Cytarabine liposome® (cytarabine liposome injection) have been reported. An overdose with Cytarabine liposome may be associated with severe chemical arachnoiditis including encephalopathy. In an early uncontrolled study without dexamethasone prophylaxis, single doses up to 125 mg were administered. There is no antidote for overdose of intrathecal Cytarabine liposome or unencapsulated cytarabine released from Cytarabine liposome. Exchange of CSF with isotonic saline has been carried out in a case of intrathecal overdose of free cytarabine, and such a procedure may be considered in the case of Cytarabine liposome overdose. Management of overdose should be directed at maintaining vital functions.
Pharmacology
There is limited information regarding Cytarabine liposome Pharmacology in the drug label.
Mechanism of Action
- Cytarabine liposome® (cytarabine liposome injection) is a sustained-release formulation of the active ingredient cytarabine designed for direct administration into the cerebrospinal fluid (CSF). Cytarabine is a cell cycle phase-specific antineoplastic agent, affecting cells only during the S-phase of cell division. Intracellularly, cytarabine is converted into cytarabine-5-triphosphate (ara-CTP), which is the active metabolite. The mechanism of action is not completely understood, but it appears that ara-CTP acts primarily through inhibition of DNA polymerase. Incorporation into DNA and RNA may also contribute to cytarabine cytotoxicity. Cytarabine is cytotoxic to proliferating mammalian cells in culture.
Structure
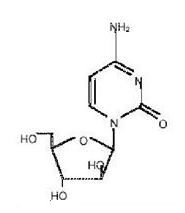
- Cytarabine liposome® (cytarabine liposome injection) is a sterile, injectable suspension of the antimetabolite cytarabine, for intrathecal administration. The chemical name of cytarabine is 4-amino-1 -β-D-arabinofuranosyl-2(1H)-pyrimidinone, and is also known as cytosine arabinoside . It has a molecular formula of C9H13N3O5, and a molecular weight 243.22 g/mol. Cytarabine has the following structural formula:
- Cytarabine liposome is available as a single-dose vial containing 50 mg/5 mL (10 mg/mL) of cytarabine. Cytarabine liposome is formulated as a sterile, non-pyrogenic, white to off-white suspension of cytarabine liposomes in 0.9% w/v sodium chloride in water for injection. Each mL contains 10 mg cytarabine, 4.4 mg cholesterol, 1.2 mg triolein, 5.7 mg dioleoylphosphatidylcholine (DOPC), and 1.0 mg dipalmitoylphosphatidylglycerol (DPPG). Cytarabine liposome is preservative-free. The pH of the product falls within the range from 5.5 to 8.5.
- Liposome drug products may behave differently from nonliposome drug products. Cytarabine liposome (cytarabine liposome injection) is not equivalent to, and cannot be substituted for, other drug products containing cytarabine.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Cytarabine liposome in the drug label.
Pharmacokinetics
- Following intrathecal administration of Cytarabine liposome 50 mg, peak levels of free CSF cytarabine were observed within 1 hour of dosing and ranged from 30 to 50 mcg/mL. The terminal half-life for the free CSF cytarabine ranged from of 5.9 to 82.4 hours. Systemic exposure to cytarabine was negligible following intrathecal administration of Cytarabine liposome 50 mg.
Metabolism and Elimination
- The primary route of elimination of cytarabine is metabolism to the inactive compound ara-U, followed by urinary excretion of ara-U. In contrast to systemically administered cytarabine, which is rapidly metabolized to ara-U, conversion to ara-U in the CSF is negligible after intrathecal administration because of the significantly lower cytidine deaminase activity in the CNS tissues and CSF. The CSF clearance rate of cytarabine is similar to the CSF bulk flow rate of 0.24 mL/min.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No carcinogenicity, mutagenicity or impairment of fertility studies have been conducted with Cytarabine liposome.
- Cytarabine was mutagenic in in vitro tests and was clastogenic in vitro (chromosome aberrations and SCE in human leukocytes) and in vivo (chromosome aberrations and SCE assay in rodent bone marrow, mouse micronucleus assay). Cytarabine caused the transformation of hamster embryo cells and rat H43 cells in vitro.
- No studies assessing the impact of cytarabine on fertility are available in the literature. Cytarabine was clastogenic to meiotic cells; a dose-dependent increase in sperm-head abnormalities and chromosomal aberrations occurred in mice given IP cytarabine. Because the systemic exposure to free cytarabine following intrathecal treatment with Cytarabine liposome was negligible, the risk of impaired fertility after intrathecal Cytarabine liposome is likely to be low.
Clinical Studies
- Cytarabine liposome® (cytarabine liposome injection) was studied in 2 controlled clinical studies that enrolled patients with neoplastic meningitis.
Study 1 – Solid Tumors, Lymphoma, or Leukemia
- The first study, which was a randomized, multi-center, multi-arm study involving a total of 99 treated patients, compared 50 mg of Cytarabine liposome administered every 2 weeks to standard intrathecal chemptherapy administered twice a week to patients with solid tumors, lymphoma, or leukemia. For patients with lymphoma, standard therapy consisted of 50 mg of unencapsulated cytarabine given twice a week. Thirty-three lymphoma patients (17 Cytarabine liposome, 16 cytarabine) were treated. Patients went off study if they had not achieved a complete response defined as clearing of the CSF from all previously positive sites in the absence of progression of neurological symptoms, after 4 weeks of treatment with study drug.
- In the first study, complete response was prospectively defined as (1) conversion, confirmed by a blinded central pathologist, from a positive examination of the CSF for malignant cells to a negative examination on two separate occasions (at least 3 days apart, on day 29 and later) at all initially positive sites, together with (2) an absence of neurological progression during the treatment period.
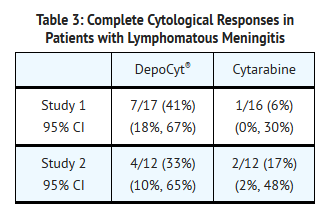
- The complete response rates in the first study of lymphoma are shown in TABLE 3. Although there was a plan for central pathology review of the data, in 4 of the 7 responding patients on the Cytarabine liposome arm this was not accomplished and these cases were considered to have had a complete response based on the reading of an unblinded pathologist. The median overall survival of all treated patients was 99.5 days in the Cytarabine liposome group and 63 days in the cytarabine group. In both groups the majority of patients died from progressive systemic disease, not neoplastic meningitis.
Study 2 – Lymphoma
- The second study was a randomized, multi-center, multi-arm study involving a total of 124 treated patients with either solid tumors or lymphomas. In this study, 24 patients with lymphoma were randomized and treated with Cytarabine liposome or cytarabine. Patients received 6 two-week induction cycles of Cytarabine liposome 50 mg every 2 weeks or cytarabine 50 mg twice weekly. Patients then received four maintenance cycles of Cytarabine liposome 50 mg every 4 weeks, or cytarabine 50 mg weekly for 4 weeks. In both studies, patients received concurrent treatment with dexamethasone to minimize symptoms associated with chemical arachnoiditis . In this study, cytological response was assessed in a blinded fashion utilizing a similar definition as in the first study. The results in patients with lymphomatous meningitis are shown in TABLE 3
How Supplied
- Cytarabine liposome® (cytarabine liposome injection) is supplied as a sterile, white to off-white suspension in 5 mL glass, single dose vials.
Storage
- Store refrigerated at 2° to 8°C (36° to 46°F). Protect from freezing and avoid aggressive agitation.
- Available in individual carton containing one ready to use vial. NDC 57665-331-01.
- Do not use beyond expiration date printed on the label.
- Cytarabine liposome is a genotoxic drug. Follow special handling and disposal procedures
Images
Drug Images
{{#ask: Page Name::Cytarabine liposome |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Cytarabine liposome |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise patients of the following expected adverse events: headache, nausea, vomiting, and fever, and about the early signs and symptoms of neurotoxicity.
- Advise patients of the importance of concurrent dexamethasone administration should be emphasized at the initiation of each cycle of Cytarabine liposome® treatment.
- Instruct patients to seek medical attention if signs or symptoms of neurotoxicity develop, or if oral dexamethasone is not well tolerated.
Precautions with Alcohol
- Alcohol-Cytarabine liposome interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Depocyt®[1]
Look-Alike Drug Names
There is limited information regarding Cytarabine liposome Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Cytarabine liposome
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Cytarabine liposome |Label Name=Cytarabine liposome 05.png
}}
{{#subobject:
|Label Page=Cytarabine liposome |Label Name=Cytarabine liposome 06.png
}}