Cholestyramine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]; Sree Teja Yelamanchili, MBBS [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Cholestyramine is a bile acid sequestrant that is FDA approved for the treatment of elevated serum cholesterol in patients with primary hypercholesterolemia (elevated low density lipoprotein [LDL] cholesterol) who do not respond adequately to diet.. Common adverse reactions include constipation.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Reduction of elevated serum cholesterol
- Dosing information
- Recommended starting adult dose: one packet or one level scoopful once or twice a day.
- Recommended maintenance dose for all: 2 to 4 packets or scoopfuls daily (8-16 grams anhydrous cholestyramine resin) divided into two doses.
- Four grams of anhydrous cholestyramine resin is contained in each measured dose of Cholestyramine as follows:

- It is recommended that increases in dose be gradual with periodic assessment of lipid/lipoprotein levels at intervals of not less than 4 weeks. The maximum recommended daily dose is six packets or scoopfuls of cholestyramine for oral suspension (24 grams of anhydrous cholestyramine resin). The suggested time of administration is at mealtime but may be modified to avoid interference with absorption of other medications. Although the recommended dosing schedule is twice daily, cholestyramine for oral suspension may be administered in 1 to 6 doses per day.
- Cholestyramine should not be taken in its dry form. Always mix Cholestyramine with water or other fluids before ingesting.
Concomitant Therapy
- Preliminary evidence suggests that the lipid-lowering effects of Cholestyramine on total and LDL-cholesterol are enhanced when combined with a HMG-CoA reductase inhibitor, e.g., pravastatin, lovastatin, simvastatin, and fluvastatin. Additive effects on LDL-cholesterol are also seen with combined nicotinic acid/Cholestyramine therapy.
Preparation
- The color of Cholestyramine may vary somewhat from batch to batch but this variation does not affect the performance of the product. Place the contents of one single-dose packet or one level scoopful of Cholestyramine in a glass or cup. Add an amount of water or other non-carbonated beverage of your choice depending on the product being used:
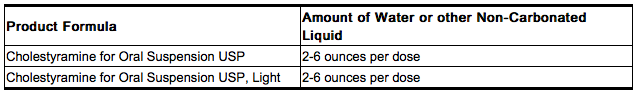
- Stir to a uniform consistency and drink.
- Cholestyramine may also be mixed with highly fluid soups or pulpy fruits with a high moisture content such as applesauce or crushed pineapple.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Cholestyramine in adult patients.
Non–Guideline-Supported Use
Bile acid malabsorption syndrome
- Dosing information
- 1 to 2 sachets TID[1]
Collagenous colitis
- Dosing information
- 4-grams (g) packets/day [2]
Fistula of bile duct
- Dosing information
- 20 g[3]
Generalized atherosclerosis
- Dosing information
- 6 g 4 times per day [4]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Reduction of elevated serum cholesterol
- Dosing information
- 240 mg/kg/day in 2-3 doses, normally not to exceed 8 gm/day with dose titration based on response and tolerance
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Cholestyramine in pediatric patients.
Non–Guideline-Supported Use
Cholestasis
- Dosing information
- Not applicable. [5]
Toddler diarrhea
- Dosing information
- 2 g BID [6]
Contraindications
Cholestyramine for oral suspension is contraindicated in patients with complete biliary obstruction where bile is not secreted into the intestine and in those individuals who have shown hypersensitivity to any of its components.
Warnings
PHENYLKETONURICS: CHOLESTYRAMINE for ORAL SUSPENSION USP, LIGHT CONTAINS 14.0 mg PHENYLALANINE PER 5 GRAM DOSE.
PRECAUTIONS
General
- Chronic use of cholestyramine resin may be associated with increased bleeding tendency due to hypoprothrombinemia associated with Vitamin K deficiency.
- This will usually respond promptly to parenteral Vitamin K1 and recurrences can be prevented by oral administration of Vitamin K1.
- Reduction of serum or red cell folate has been reported over long term administration of cholestyramine resin.
- Supplementation with folic acid should be considered in these cases.
- There is a possibility that prolonged use of cholestyramine resin, since it is a chloride form of anion exchange resin, may produce hyperchloremic acidosis.
- This would especially be true in younger and smaller patients where the relative dosage may be higher.
- Caution should also be exercised in patients with renal insufficiency or volume depletion, and in patients receiving concomitant spironolactone.
- Cholestyramine resin may produce or worsen pre-existing constipation. *The dosage should be increased gradually in patients to minimize the risk of developing fecal impaction.
- In patients with pre-existing constipation, the starting dose should be 1 packet or 1 scoop once daily for 5 to 7 days, increasing to twice daily with monitoring of constipation and of serum lipoproteins, at least twice, 4 to 6 weeks apart.
- Increased fluid intake and fiber intake should be encouraged to alleviate constipation and a stool softener may occasionally be indicated.
- If the initial dose is well tolerated, the dose may be increased as needed by one dose/day (at monthly intervals) with periodic monitoring of serum lipoproteins.
- If constipation worsens or the desired therapeutic response is not achieved at one to six doses/day, combination therapy or alternate therapy should be considered.
- Particular effort should be made to avoid constipation in patients with symptomatic coronary artery disease.
- Constipation associated with cholestyramine resin may aggravate hemorrhoids.
Laboratory Tests
Serum cholesterol levels should be determined frequently during the first few months of therapy and periodically thereafter. Serum triglyceride levels should be measured periodically to detect whether significant changes have occurred. The LRC-CPPT showed a dose-related increase in serum triglycerides of 10.7%–17.1% in the cholestyramine-treated group, compared with an increase of 7.9%–11.7% in the placebo group. Based on the mean values and adjusting for the placebo group, the cholestyramine-treated group showed an increase of 5% over pre-entry levels the first year of the study and an increase of 4.3% the seventh year.
Adverse Reactions
Clinical Trials Experience
- The most common adverse reaction is constipation.
- When used as a cholesterol-lowering agent predisposing factors for most complaints of constipation are high dose and increased age (more than 60 years old).
- Most instances of constipation are mild, transient, and controlled with conventional therapy.
- Some patients require a temporary decrease in dosage or discontinuation of therapy.
Less Frequent Adverse Reactions
- Abdominal discomfort and/or pain, flatulence, nausea, vomiting, diarrhea, eructation, anorexia, and steatorrhea, bleeding tendencies due to hypoprothrombinemia (Vitamin K deficiency) as well as Vitamin A (one case of night blindness reported) and D deficiencies, hyperchloremic acidosis in children, osteoporosis, rash and irritation of the skin, tongue and perianal area. Rare reports of intestinal obstruction, including two deaths, have been reported in pediatric patients.
- Occasional calcified material has been observed in the biliary tree, including calcification of the gallbladder, in patients to whom cholestyramine resin has been given.
- However, this may be a manifestation of the liver disease and not drug related.
- One patient experienced biliary colic on each of three occasions on which he took cholestyramine resin.
- One patient diagnosed as acute abdominal symptom complex was found to have a “pasty mass” in the transverse colon on x-ray.
- Other events (not necessarily drug related) reported in patients taking cholestyramine resin include:
Gastrointestinal—GI-rectal bleeding, black stools, hemorrhoidal bleeding, bleeding from known duodenal ulcer, dysphagia, hiccups, ulcer attack, sour taste, pancreatitis, rectal pain, diverticulitis.
Laboratory test changes—Liver function abnormalities.
Hematologic—Prolonged prothrombin time, ecchymosis, anemia.
Hypersensitivity—Urticaria, asthma, wheezing, shortness of breath.
Musculoskeletal—Backache, muscle and joint pains, arthritis.
Neurologic—Headache, anxiety, vertigo, dizziness, fatigue, tinnitus, syncope, drowsiness, femoral nerve pain, paresthesia.
Eye—Uveitis.
Renal—Hematuria, dysuria, burnt odor to urine, diuresis.
Miscellaneous—Weight loss, weight gain, increased libido, swollen glands, edema, dental bleeding, dental caries, erosion of tooth enamel, tooth discoloration.
Postmarketing Experience
There is limited information about the Post marketing Experience.
Drug Interactions
- Cholestyramine for Oral Suspension USP may delay or reduce the absorption of concomitant oral medication such as phenylbutazone, warfarin, thiazide diuretics (acidic), or propranolol (basic), as well as tetracycline, penicillin G, phenobarbital, thyroid and thyroxine preparations, estrogens and progestins, and digitalis. *Interference with the absorption of oral phosphate supplements has been observed with another positively-charged bile acid sequestrant. *Cholestyramine may interfere with the pharmacokinetics of drugs that undergo entero-hepatic circulation.
- The discontinuance of Cholestyramine could pose a hazard to health if a potentially toxic drug such as digitalis has been titrated to a maintenance level while the patient was taking Cholestyramine.
- Because cholestyramine binds bile acids, Cholestyramine may interfere with normal fat digestion and absorption and thus may prevent absorption of fat-soluble vitamins such as A, D, E and K.
- When Cholestyramine is given for long periods of time, concomitant supplementation with water-miscible (or parenteral) forms of fat-soluble vitamins should be considered.
- SINCE CHOLESTYRAMINE MAY BIND OTHER DRUGS GIVEN CONCURRENTLY, IT IS RECOMMENDED THAT PATIENTS TAKE OTHER DRUGS AT LEAST ONE HOUR BEFORE OR 4 TO 6 HOURS AFTER CHOLESTYRAMINE (OR AT AS GREAT AN INTERVAL AS POSSIBLE) TO AVOID IMPEDING THEIR ABSORPTION.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
There are no adequate and well controlled studies in pregnant women. The use of Cholestyramine in pregnancy or lactation or by women of childbearing age requires that the potential benefits of drug therapy be weighed against the possible hazards to the mother and child. Cholestyramine is not absorbed systemically, however, it is known to interfere with absorption of fat-soluble vitamins; accordingly, regular prenatal supplementation may not be adequate.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cholestyramine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Cholestyramine during labor and delivery.
Nursing Mothers
Caution should be exercised when Cholestyramine is administered to a nursing mother. The possible lack of proper vitamin absorption described in the “Pregnancy” section may have an effect on nursing infants.
Pediatric Use
Although an optimal dosage schedule has not been established, standard texts (6,7) list a usual pediatric dose of 240 mg/kg/day of anhydrous cholestyramine resin in two to three divided doses, normally not to exceed 8 gm/day with dose titration based on response and tolerance. In calculating pediatric dosages, 44.4 mg of anhydrous cholestyramine resin are contained in 100 mg of Cholestyramine for Oral Suspension USP and 80 mg of anhydrous cholestyramine resin are contained in 100 mg of Cholestyramine for Oral Suspension USP, Light. The effects of long-term administration, as well as its effect in maintaining lowered cholesterol levels in pediatric patients, are unknown.
Geriatic Use
There is no FDA guidance on the use of Cholestyramine in geriatric settings.
Gender
There is no FDA guidance on the use of Cholestyramine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cholestyramine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Cholestyramine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Cholestyramine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Cholestyramine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Cholestyramine in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
There is limited information about the drug monitoring.
IV Compatibility
There is limited information about the IV Compatibility.
Overdosage
Overdosage with Cholestyramine has been reported in a patient taking 150% of the maximum recommended daily dosage for a period of several weeks. No ill effects were reported. Should an overdosage occur, the chief potential harm would be obstruction of the gastrointestinal tract. The location of such potential obstruction, the degree of obstruction, and the presence or absence of normal gut motility would determine treatment.
Pharmacology
| |
Cholestyramine
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | C10 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | ? |
| Mol. mass | Average MW exceeds 106 Daltons |
| Pharmacokinetic data | |
| Bioavailability | low |
| Protein binding | unknown |
| Metabolism | bile acids |
| Half life | .1 hr |
| Excretion | feces |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) C |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) Template:Unicode Prescription only |
| Routes | oral |
Mechanism of Action
There is limited information about the mechanism of action.
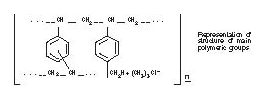
Structure
Cholestyramine for Oral Suspension USP, the chloride salt of a basic anion exchange resin, a cholesterol lowering agent, is intended for oral administration. Cholestyramine resin is quite hydrophilic, but insoluble in water. The cholestyramine resin in Cholestyramine is not absorbed from the digestive tract. Four grams of anhydrous cholestyramine resin is contained in 9 grams of Cholestyramine for Oral Suspension USP. Four grams of anhydrous cholestyramine resin is contained in 5 grams of Cholestyramine for Oral Suspension USP, Light. It is represented by the following structural formula:
Cholestyramine for Oral Suspension USP contains the following inactive ingredients: acacia, citric acid, D&C Yellow No. 10, FD&C Yellow No. 6, flavor (natural and artificial Orange), polysorbate 80, propylene glycol alginate and sucrose. Cholestyramine for Oral Suspension USP, Light contains the following inactive ingredients: aspartame, citric acid, colloidal silicon dioxide, D&C Yellow No. 10, FD&C Red No. 40, flavor (natural and artificial Orange), maltodextrin, propylene glycol alginate and xanthan gum.
Pharmacodynamics
There is limited information about the pharmacodynamics.
Pharmacokinetics
There is limited information about the pharmacokinetics.
Nonclinical Toxicology
In studies conducted in rats in which cholestyramine resin was used as a tool to investigate the role of various intestinal factors, such as fat, bile salts and microbial flora, in the development of intestinal tumors induced by potent carcinogens, the incidence of such tumors was observed to be greater in cholestyramine resin-treated rats than in control rats. The relevance of this laboratory observation from studies in rats to the clinical use of Cholestyramine is not known. In the LRC-CPPT study referred to above, the total incidence of fatal and nonfatal neoplasms was similar in both treatment groups. When the many different categories of tumors are examined, various alimentary system cancers were somewhat more prevalent in the cholestyramine group. The small numbers and the multiple categories prevent conclusions from being drawn. However, in view of the fact that cholestyramine resin is confined to the GI tract and not absorbed, and in light of the animal experiments referred to above, a six-year post-trial follow-up of the LRC-CPPT 5 patient population has been completed (a total of 13.4 years of in-trial plus post-trial follow-up) and revealed no significant difference in the incidence of cause-specific mortality or cancer morbidity between cholestyramine and placebo treated patients.
Clinical Studies
In a large, placebo-controlled, multi-clinic study, LRC-CPPT1 , hypercholesterolemic subjects treated with cholestyramine had mean reductions in total and low-density lipoprotein cholesterol (LDL-C) which exceeded those for diet and placebo treatment by 7.2% and 10.4 %, respectively. Over the seven-year study period the Cholestyramine group experienced a 19% reduction (relative to the incidence in the placebo group) in the combined rate of coronary heart disease death plus non-fatal myocardial infarction (cumulative incidences of 7% Cholestyramine and 8.6% placebo). The subjects included in the study were men aged 35-59 with serum cholesterol levels above 265 mg/dL and no previous history of heart disease. It is not clear to what extent these findings can be extrapolated to females and other segments of the hypercholesterolemic population. Two controlled clinical trials have examined the effects of Cholestyramine monotherapy upon coronary atherosclerotic lesions using coronary arteriography. In the NHLBI Type II Coronary Intervention Trial2, 116 patients (80% male) with coronary artery disease (CAD) documented by arteriography were randomized to Cholestyramine or placebo for five years of treatment. Final study arteriography revealed progression of coronary artery disease in 49% of placebo patients compared to 32% of the Cholestyramine group (p<0.05). In the St. Thomas Atherosclerosis Regression Study (STARS)3, 90 hypercholesteroleic men with CAD were randomized to three blinded treatments: usual care, lipid-lowering diet, and lipid-lowering diet plus Cholestyramine. After 36 months, follow-up coronary arteriography revealed progression of disease in 46% of usual care patients, 15% of patients on lipid-lowering diet and 12% of those receiving diet plus Cholestyramine (p<0.02). The mean absolute width of coronary segments decreased in the usual care group, increased slightly (0.003mm) in the diet group and increased by 0.103mm in the diet plus Cholestyramine group (p<0.05). Thus in these randomized controlled clinical trials using coronary arteriography, Cholestyramine monotherapy has been demonstrated to slow progression2,3 and promote regression3 of atherosclerotic lesions in the coronary arteries of patients with coronary artery disease. The effect of intensive lipid-lowering therapy on coronary atherosclerosis has been assessed by arteriography in hyperlipidemic patients. In these randomized, controlled clinical trials, patients were treated for two to four years by either conventional measures (diet, placebo, or in some cases low dose resin), or intensive combination therapy using diet plus colestipol (an anion exchange resin with a mechanism of action and an effect on serum lipids similar to that of Cholestyramine for Oral Suspension USP and Cholestyramine for Oral Suspension USP, Light) plus either nicotinic acid or lovastatin. When compared to conventional measures, intensive lipid-lowering combination therapy significantly reduced the frequency of progression and increased the frequency of regression of coronary atherosclerotic lesions in patients with or at risk for coronary artery disease.
How Supplied
Cholestyramine for Oral Suspension USP is a yellow colored orange flavored powder available in cans containing 378 grams and in cartons of sixty 9 gram packets. Four grams of anhydrous cholestyramine resin are contained in 9 grams of Cholestyramine for Oral Suspension USP. The 378 g can includes a 15 cc scoop. The scoop is not interchangeable with scoops from other products. Made in U.S.A.

Cholestyramine for Oral Suspension USP, Light is a cream to pale yellow colored orange flavored powder available in cans containing 210 grams and in cartons of sixty 5 gram packets. Four grams of anhydrous cholestyramine resin are contained in 5 grams of Cholestyramine for Oral Suspension USP, Light. The 210 g can includes a 9 cc scoop. The scoop is not interchangeable with scoops from other products. Made in India*

Storage
Store between 20º to 25ºC (68º to 77ºF). Excursions permitted to 15º to 30ºC (59º to 86ºF).
Images
Drug Images
{{#ask: Page Name::Cholestyramine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Cholestyramine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Inform your physician if you are pregnant or plan to become pregnant or are breastfeeding. Drink plenty of fluids and mix each 9 gram dose of Cholestyramine for Oral Suspension USP in at least 2 to 6 ounces of fluid. Mix each 5 gram dose of Cholestyramine for Oral Suspension USP, Light in at least 2 to 6 ounces of fluid before taking. Sipping or holding the resin suspension in the mouth for prolonged periods may lead to changes in the surface of the teeth resulting in discoloration, erosion of enamel or decay; good oral hygiene should be maintained.
Precautions with Alcohol
Alcohol-Cholestyramine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Cholestyramine Brand Names in the drug label.
Look-Alike Drug Names
There is limited information about the Look-Alike Drug Names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Sinha L, Liston R, Testa HJ, Moriarty KJ (1998). "Idiopathic bile acid malabsorption: qualitative and quantitative clinical features and response to cholestyramine". Aliment Pharmacol Ther. 12 (9): 839–44. PMID 9768525.
- ↑ Ung KA, Gillberg R, Kilander A, Abrahamsson H (2000). "Role of bile acids and bile acid binding agents in patients with collagenous colitis". Gut. 46 (2): 170–5. PMC 1727822. PMID 10644309.
- ↑ Bell SN, Varigos GA (1980). "Treatment of skin irritations around biliary fistulas with cholestyramine". Br J Surg. 67 (11): 785. PMID 7427037.
- ↑ Brensike JF, Levy RI, Kelsey SF, Passamani ER, Richardson JM, Loh IK; et al. (1984). "Effects of therapy with cholestyramine on progression of coronary arteriosclerosis: results of the NHLBI Type II Coronary Intervention Study". Circulation. 69 (2): 313–24. PMID 6360414.
- ↑ Deutsch J, Smith AL, Danks DM, Campbell PE (1985). "Long term prognosis for babies with neonatal liver disease". Arch Dis Child. 60 (5): 447–51. PMC 1777337. PMID 2990356.
- ↑ Vesikari T, Isolauri E (1985). "A comparative trial of cholestyramine and loperamide for acute diarrhoea in infants treated as outpatients". Acta Paediatr Scand. 74 (5): 650–4. PMID 3901661.
{{#subobject:
|Page Name=Cholestyramine
|Pill Name=
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Cholestyramine |Label Name=Cholestyramine_label_01.jpg
}}
{{#subobject:
|Label Page=Cholestyramine |Label Name=Cholestyramine_label_02.jpg
}}
{{#subobject:
|Label Page=Cholestyramine |Label Name=Cholestyramine_label_03.jpg
}}
{{#subobject:
|Label Page=Cholestyramine |Label Name=Cholestyramine_label_04.jpg
}}
{{#subobject:
|Label Page=Cholestyramine |Label Name=Cholestyramine_label_05.jpg
}}
{{#subobject:
|Label Page=Cholestyramine |Label Name=Cholestyramine_label_06.jpg
}}
{{#subobject:
|Label Page=Cholestyramine |Label Name=Cholestyramine_panel_01.png
}}
{{#subobject:
|Label Page=Cholestyramine |Label Name=Cholestyramine_panel_02.png
}}
{{#subobject:
|Label Page=Cholestyramine |Label Name=Cholestyramine_panel_03.png
}}