Ceftibuten microbiology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
Ceftibuten exerts its bactericidal action by binding to essential target proteins of the bacterial cell wall. This binding leads to inhibition of cell-wall synthesis.
Ceftibuten is stable in the presence of most plasmid-mediated beta-lactamases, but it is not stable in the presence of chromosomally-mediated cephalosporinases produced in organisms such as Bacteroides, Citrobacter, Enterobacter, Morganella, and Serratia. Like other beta-lactam agents, ceftibuten should not be used against strains resistant to beta-lactams due to general mechanisms such as permeability or penicillin-binding protein changes like penicillin-resistant S. pneumoniae.
Ceftibuten has been shown to be active against most strains of the following organisms both in vitro and in clinical infections (see INDICATIONS AND USAGE):
Gram-positive aerobes:
Streptococcus pneumoniae (penicillin-susceptible strains only)
Gram-negative aerobes:
Haemophilus influenzae (including β-lactamase-producing strains)
Moraxella catarrhalis (including β-lactamase-producing strains)
There are no known organisms which are potential pathogens in the indications approved for ceftibuten for which ceftibuten exhibits in vitro activity but for which the safety and efficacy of ceftibuten in treating clinical infections due to these organisms, have not been established in adequate and well-controlled trials.
NOTE: Ceftibuten is INACTIVE in vitro against Acinetobacter,Bordetella, Campylobacter, Enterobacter, Enterococcus, Flavobacterium, Hafnia, Listeria, Pseudomonas, Staphylococcus, and Streptococcus (except pneumoniae and pyogenes) species. In addition, it shows little in vitro activity against most anaerobes, including most species of Bacteroides.
Susceptibility Testing
Dilution Techniques
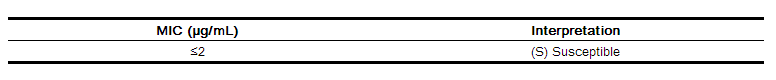
Quantitative methods are used to determine antimicrobial minimal inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method (broth, agar, or microdilution) or equivalent with standardized inoculum concentrations and standardized concentrations of ceftibuten powder. The MIC values should be interpreted according to the following criteria when testing Haemophilus species using Haemophilus Test Media (HTM):
The current absence of resistant strains precludes defining any categories other than "Susceptible". Strains yielding results suggestive of a "Nonsusceptible" category should be submitted to a reference laboratory for further testing.
A report of "Susceptible" implies that an infection due to the strain may be appropriately treated with the dosage of antimicrobial agent recommended for that type of infection and infecting species, unless otherwise contraindicated.
Ceftibuten is indicated for penicillin-susceptible only strains of Streptococcus pneumoniae. A pneumococcal isolate that is susceptible to penicillin (MIC ≤0.06 µg/mL) can be considered susceptible to ceftibuten for approved indications. Testing of ceftibuten against penicillin-intermediate or penicillin resistant isolates is not recommended. Reliable interpretive criteria for ceftibuten are not currently available. Physicians should be informed that clinical response rates with ceftibuten may be lower in strains that are not penicillin-susceptible.
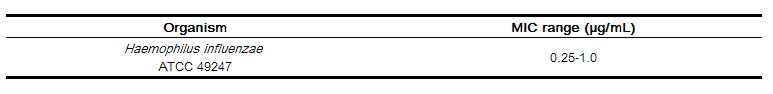
Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspect of laboratory procedures. Standard ceftibuten powder should provide the following MIC values:
Diffusion Techniques
Quantitative methods that require measurement of zone diameters also provide estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 30 µg of ceftibuten to test the susceptibility of microorganisms to ceftibuten.
Reports from the laboratory providing results of the standard single-disk susceptibility test with a 30-µg ceftibuten disk should be interpreted according to the following criteria when testing Haemophilus species using Haemophilus Test Media (HTM):
The current absence of resistant strains precludes defining any categories other than "Susceptible". Strains yielding results suggestive of a "Nonsusceptible" category should be submitted to a reference laboratory for further testing.
Interpretation should be as stated above for results using dilution techniques.
Ceftibuten is indicated for penicillin-susceptible only strains of Streptococcus pneumoniae. Pneumococcal isolates with oxacillin zone sizes of ≥20 mm are susceptible to penicillin and can be considered susceptible for approved indications. Reliable disk diffusion tests for ceftibuten do not yet exist.
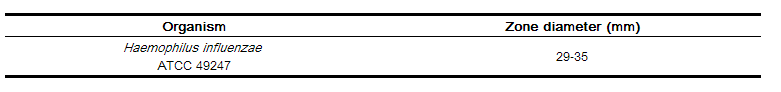
As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 30-µg ceftibuten disk should provide the following zone diameters in these laboratory test quality control strains:
References
http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/050686s016lbl.pdf