Amygdala
| File:Wiktionary-logo-en-v2.svg | Look up amygdala in Wiktionary, the free dictionary. |
The amygdalae (Latin, also corpus amygdaloideum, singular amygdala, from Greek αμυγδαλή, amygdalē, 'almond', 'tonsil')[1] are almond-shaped groups of neurons located deep within the medial temporal lobes of the brain in complex vertebrates, including humans. [2] Shown in research to perform a primary role in the processing and memory of emotional reactions, the amygdalae are considered part of the limbic system. [3]
Anatomical subdivisions
The regions described as amygdalae encompass several nuclei with distinct functional traits. Among these nuclei are the basolateral complex, the centromedial nucleus and the cortical nucleus. The basolateral complex can be further subdivided into the lateral, the basal and the accessory basal nuclei. [3][4]
Connections
The amygdalae send impulses to the hypothalamus for important activation of the sympathetic nervous system, to the thalamic reticular nucleus for increased reflexes, to the nuclei of the trigeminal nerve and facial nerve for facial expressions of fear, and to the ventral tegmental area, locus coeruleus, and laterodorsal tegmental nucleus for activation of dopamine, norepinephrine and epinephrine.[4]
The cortical nucleus is involved in the sense of smell and pheromone-processing. It receives input from the olfactory bulb and olfactory cortex. The lateral amygdalae, which send impulses to the rest of the basolateral complexes and to the centromedial nuclei, receive input from the sensory systems. The centromedial nuclei are the main outputs for the basolateral complexes, and are involved in emotional arousal in rats and cats.[4][5]
Emotional learning
In complex vertebrates, including humans, the amygdala perform primary roles in the formation and storage of memories associated with emotional events. Research indicates that during fear conditioning, sensory stimuli reach the basolateral complexes of the amygdalae, particularly the lateral nuclei, where they form associations with memories of the stimuli. The association between stimuli and the aversive events they predict may be mediated by long-term potentiation, a lingering potential for affected synapses to react more readily.[3]
Memories of emotional experiences imprinted in reactions of synapses in the lateral nuclei elicit fear behavior through connections with the central nucleus of the amygdalae. The central nuclei are involved in the genesis of many fear responses, including freezing (immobility), tachycardia (rapid heartbeat), increased respiration, and stress-hormone release. Damage to the amygdalae impairs both the acquisition and expression of Pavlovian fear conditioning, a form of classical conditioning of emotional responses.[3]
The amygdalae are also involved in appetitive (positive) conditioning. It seems that distinct neurons respond to positive and negative stimuli, but there is no clustering of these distinct neurons into clear anatomical nuclei.[6]
Different nuclei within the amygdala have different functions in appetitive conditioning.[7]
Memory modulation
The amygdalae also are involved in the modulation of memory consolidation. Following any learning event, the long-term memory for the event is not instantaneously formed. Rather, information regarding the event is slowly assimilated into long-term storage over time (the duration of long-term memory storage can be infinite), a process referred to as memory consolidation, until it reaches a relatively permanent state.
During the consolidation period, the memory can be modulated. In particular, it appears that emotional arousal following the learning event influences the strength of the subsequent memory for that event. Greater emotional arousal following a learning event enhances a person's retention of that event. Experiments have shown [1] that administration of stress hormones to mice immediately after they learn something enhances their retention when they are tested two days later.
The amygdalae, especially the basolateral nuclei, are involved in mediating the effects of emotional arousal on the strength of the memory for the event, as shown by many laboratories including that of James McGaugh. These laboratories have trained animals on a variety of learning tasks and found that drugs injected into the amygdala after training affect the animals' subsequent retention of the task. These tasks include basic classical conditioning tasks such as inhibitory avoidance, where a rat learns to associate a mild footshock with a particular compartment of an apparatus, and more complex tasks such as spatial or cued water maze, where a rat learns to swim to a platform to escape the water. If a drug that activates the amygdalae is injected into the amygdalae, the animals had better memory for the training in the task.[8] If a drug that inactivates the amygdalae is injected, the animals had impaired memory for the task.
Despite the importance of the amygdalae in modulating memory consolidation, however, learning can occur without it, though such learning appears to be impaired, as in fear conditioning impairments following amygdalar damage.[9]
Evidence from work with humans indicates that the amygdala plays a similar role. Amygdala activity at the time of encoding information correlates with retention for that information. However, this correlation depends on the relative "emotionalness" of the information. More emotionally-arousing information increases amygdalar activity, and that activity correlates with retention.[citation needed]
Neuropsychological correlates of amygdala activity
Early research on primates provided explanations as to the functions of the amygdala, as well as a basis for further research. As early as 1888, rhesus monkeys with a lesioned temporal cortex (including the amygdala) were observed to have significant social and emotional deficits.[10] Heinrich Klüver and Paul Bucy later expanded upon this same observation by showing that large lesions to the anterior temporal lobe produced noticeable changes, including overreaction to all objects, hypoemotionality, loss of fear, hypersexuality, and hyperorality, a condition in which inappropriate objects are placed in the mouth. Some monkeys also displayed an inability to recognize familiar objects and would approach animate and inanimate objects indiscriminately, exhibiting a loss of fear towards the experimenters. This behavioral disorder was later named Klüver-Bucy syndrome accordingly.[11] Later studies served to focus on the amygdala specifically, as the temporal cortex encompasses a broad set of brain structures, making it difficult to find which ones specifically may have correlated with certain symptoms. Monkey mothers who had amygdala damage showed a reduction in maternal behaviors towards their infants, often physically abusing or neglecting them.[12] In 1981, researchers found that selective radio frequency lesions of the whole amygdala caused Klüver-Bucy Syndrome.[13]
With advances in neuroimaging technology such as MRI, neuroscientists have made significant findings concerning the amygdala in the human brain. Consensus of data shows the amygdala has a substantial role in mental states, and is related to many psychological disorders. In a 2003 study, subjects with Borderline Personality Disorder showed significantly greater left amygdala activity than normal control subjects. Some borderline patients even had difficulties classifying neutral faces or saw them as threatening.[14] In 2006, researchers observed hyperactivity in the amygdala when patients were shown threatening faces or confronted with frightening situations. Patients with more severe social phobia showed a correlation with increased response in the amygdala.[15] Similarly, depressed patients showed exaggerated left amygdala activity when interpreting emotions for all faces, and especially for fearful faces. Interestingly, this hyperactivity was normalized when patients went on antidepressants.[16] By contrast, the amygdala has been observed to relate differently in people with Bipolar Disorder. A 2003 study found that adult and adolescent bipolar patients tended to have considerably smaller amygdala volumes and somewhat smaller hippocampal volumes.[17] Many studies have focused on the connections between the amygdala and autism.[18]
Recent research suggests that parasites, in particular toxoplasma, form cysts in the brain, often taking up residence in the amygdala. This may provide clues as to how specific parasites manipulate behavior and may contribute to the development of disorders, including paranoia.[19]
Additional images
-
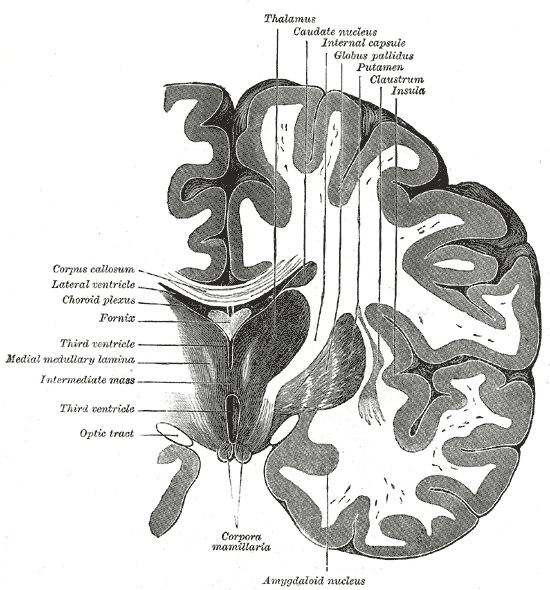
Coronal section of brain through intermediate mass of third ventricle.
-
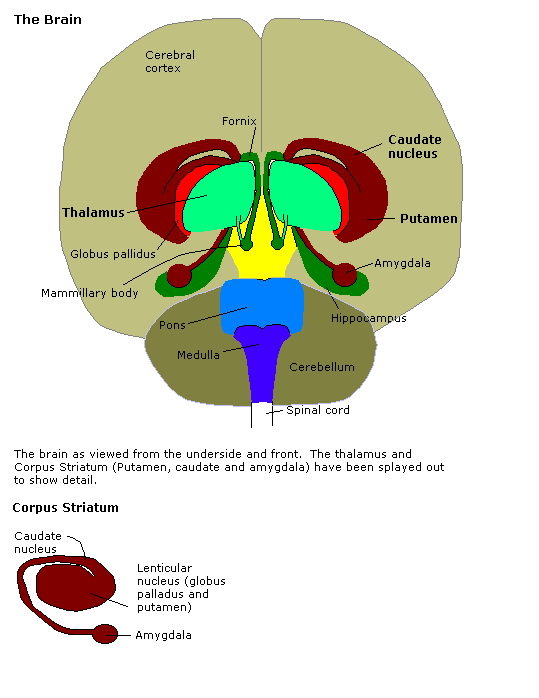
Brain
See also
References
- ↑ http://dictionary.reference.com/search?q=amygdala
- ↑ University of Idaho College of Science (2004). "amygdala". Retrieved 2007-03-15.
- ↑ 3.0 3.1 3.2 3.3 Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah N, Habel U, Schneider F, Zilles K (2005). "Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps". Anat Embryol (Berl). 210 (5–6): 343–52. PMID 16208455.
- ↑ 4.0 4.1 4.2 Ben Best (2004). "The Amygdala and the Emotions". Retrieved 2007-03-15.
- ↑ Michael McDannald, Erin Kerfoot, Michela Gallagher, and Peter C. Holland, John Hopkins University (2005). "Amygdala central nucleus function is necessary for learning but not expression of conditioned visual orienting". Retrieved 2007-03-15.
- ↑ Patton, Joseph (25 November 2005). "The primate amygdala represents the positive and negative value of visual stimuli during learning". Nature. 439: 865–870. doi:10.1038/nature04490. Unknown parameter
|coauthors=ignored (help) - ↑ See recent TINS article by Balleine and Killcross (2006)
- ↑ Ferry B, Roozendaal B, McGaugh J (1999). "Role of norepinephrine in mediating stress hormone regulation of long-term memory storage: a critical involvement of the amygdala". Biol Psychiatry. 46 (9): 1140–52. PMID 10560021.
- ↑ Killcross S, Robbins T, Everitt B (1997). "Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala". Nature. 388 (6640): 377–80. PMID 9237754.
- ↑ Brown, S. & Shafer, E. (1888). "An investigation into the functions of the occipital and temporal lobes of the monkey's brain". Philosophical Transactions of the Royal Society of London: Biological Sciences. 179: 303–327.
- ↑ Kluver, H. & Bucy, P. (1939). "Preliminary analysis of function of the temporal lobe in monkeys". Archives of Neurology. 42: 979–1000.
- ↑ Bucher, K., Myersn, R., Southwick, C. (1970). "Anterior temporal cortex and maternal behaviour in monkey". Neurology. 20: 415.
- ↑ Aggleton, JP. & Passingham, RE. (1981). "Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta)". Journal of Comparative and Physiological Psychology. 95: 961–977.
- ↑ Donegan; et al. (2003). "Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation". Biological Psychiatry. 54 (11): 1284–1293.
- ↑ Studying Brain Activity Could Aid Diagnosis Of Social Phobia. Monash University. January 19, 2006.
- ↑ Sheline; et al. (2001). "Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study". Biological Psychiatry. 50 (9): 651–658.
- ↑ Blumberg; et al. (2003). "Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder". Arch Gen Psychiatry. 60 (12): 1201–8. PMID 14662552.
- ↑ Schultz RT (2005). "Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area". Int J Dev Neurosci. 23 (2–3): 125–41. doi:10.1016/j.ijdevneu.2004.12.012. PMID 15749240.
- ↑ Vyas; et al. (2007). "Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors". Proc Natl Acad Sci U S A. 104 (15): 6442–7. PMID 17404235.
External links
Template:Limbic system Template:Prosencephalon
da:Amygdala de:Amygdala hr:Amigdala it:Amigdala he:אמיגדלה nl:Amygdala no:Amygdala sv:Amygdala
- Pages with reference errors
- CS1 maint: Multiple names: authors list
- Pages with citations using unsupported parameters
- CS1 maint: Explicit use of et al.
- Pages with broken file links
- Articles containing Latin-language text
- Articles containing Greek-language text
- Articles containing Ancient Greek-language text
- All articles with unsourced statements
- Articles with unsourced statements from February 2007
- Articles with invalid date parameter in template
- Cerebrum
- Neuroanatomy
- Limbic system