Torsemide (tablet)
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a601212 |
| Pregnancy category |
|
| Routes of administration | Oral, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 80-90% |
| Protein binding | Highly bound (>99%). |
| Metabolism | Hepatic (80%) |
| Elimination half-life | 3.5 hours; Cirrhosis: 7-8 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
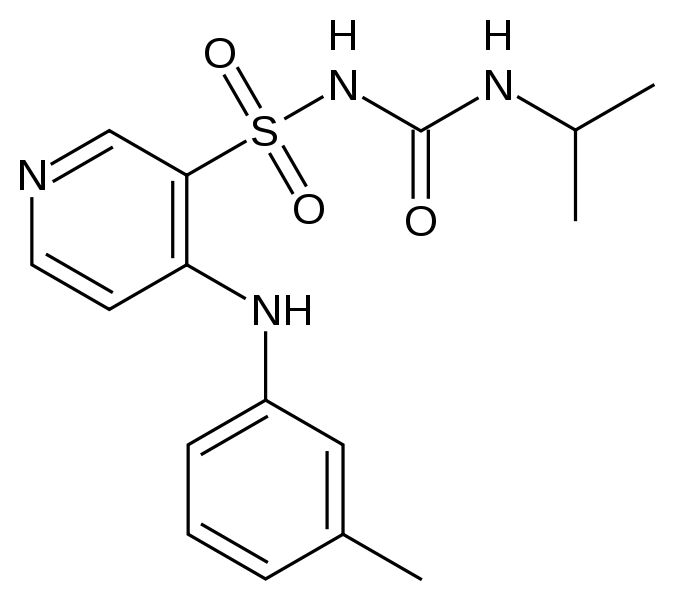
| Formula | C16H20N4O3S |
| Molar mass | 348.421 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Synonyms / Brand Names: Demadex®; generic
Overview
Torsemide is a diuretic of the pyridine-sulfonylurea class used in treatment of edema associated with congestive heart failure, renal disease, or hepatic disease.
Category
Loop diuretic