Ticlopidine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alejandro Lemor, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning
See full prescribing information for complete Boxed Warning.
Ticlopidine can cause life-threatening hematological adverse reactions, including neutropenia/agranulocytosis, thrombotic thrombocytopenic purpura (TTP) and aplastic anemia.
Neutropenia/Agranulocytosis: Among 2048 patients in clinical trials in stroke patients, there were 50 cases (2.4%) of neutropenia (less than 1200 neutrophils/mm3), and the neutrophil count was below 450/mm3 in 17 of these patients (0.8% of the total population). TTP:One case of thrombotic thrombocytopenic purpura was reported during clinical trials in stroke patients. Based on postmarketing data, US physicians reported about 100 cases between 1992 and 1997. Based on an estimated patient exposure of 2 million to 4 million, and assuming an event reporting rate of 10% (the true rate is not known), the incidence of ticlopidine-associated TTP may be as high as one case in every 2000 to 4000 patients exposed. Aplastic Anemia:Aplastic anemia was not seen during clinical trials in stroke patients, but US physicians reported about 50 cases between 1992 and 1998. Based on an estimated patient exposure of 2 million to 4 million, and assuming an event reporting rate of 10% (the true rate is not known), the incidence of ticlopidine-associated aplastic anemia may be as high as one case in every 4000 to 8000 patients exposed. Monitoring of Clinical and Hematologic Status: Severe hematological adverse reactions may occur within a few days of the start of therapy. The incidence of TTP peaks after about 3 to 4 weeks of therapy and neutropenia peaks at approximately 4 to 6 weeks. The incidence of aplastic anemia peaks after about 4 to 8 weeks of therapy. The incidence of the hematologic adverse reactions declines thereafter. Only a few cases of neutropenia, TTP, or aplastic anemia have arisen after more than 3 months of therapy. Hematological adverse reactions cannot be reliably predicted by any identified demographic or clinical characteristics. During the first 3 months of treatment, patients receiving ticlopidine must, therefore, be hematologically and clinically monitored for evidence of neutropenia or TTP. If any such evidence is seen, ticlopidine should be immediately discontinued. |
Overview
Ticlopidine is a platelet aggregation inhibitor that is FDA approved for the prophylaxis of thromboembolic stroke, and subacute stent thrombosis in patients undergoing successful coronary stent implantation.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include rash, abdominal pain, diarrhea, indigestion, loss of appetite, nausea, hemorrhage, leukopenia, dizziness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
- Hypersensitivity to the drug
- Presence of hematopoietic disorders such as neutropenia and thrombocytopenia or a past history of either TTP or aplastic anemia.
- Presence of a hemostatic disorder or active pathological bleeding (such as bleeding peptic ulcer or intracranial bleeding)
- Patients with severe liver impairment
Warnings
|
Warning
See full prescribing information for complete Boxed Warning.
Ticlopidine can cause life-threatening hematological adverse reactions, including neutropenia/agranulocytosis, thrombotic thrombocytopenic purpura (TTP) and aplastic anemia.
Neutropenia/Agranulocytosis: Among 2048 patients in clinical trials in stroke patients, there were 50 cases (2.4%) of neutropenia (less than 1200 neutrophils/mm3), and the neutrophil count was below 450/mm3 in 17 of these patients (0.8% of the total population). TTP:One case of thrombotic thrombocytopenic purpura was reported during clinical trials in stroke patients. Based on postmarketing data, US physicians reported about 100 cases between 1992 and 1997. Based on an estimated patient exposure of 2 million to 4 million, and assuming an event reporting rate of 10% (the true rate is not known), the incidence of ticlopidine-associated TTP may be as high as one case in every 2000 to 4000 patients exposed. Aplastic Anemia:Aplastic anemia was not seen during clinical trials in stroke patients, but US physicians reported about 50 cases between 1992 and 1998. Based on an estimated patient exposure of 2 million to 4 million, and assuming an event reporting rate of 10% (the true rate is not known), the incidence of ticlopidine-associated aplastic anemia may be as high as one case in every 4000 to 8000 patients exposed. Monitoring of Clinical and Hematologic Status: Severe hematological adverse reactions may occur within a few days of the start of therapy. The incidence of TTP peaks after about 3 to 4 weeks of therapy and neutropenia peaks at approximately 4 to 6 weeks. The incidence of aplastic anemia peaks after about 4 to 8 weeks of therapy. The incidence of the hematologic adverse reactions declines thereafter. Only a few cases of neutropenia, TTP, or aplastic anemia have arisen after more than 3 months of therapy. Hematological adverse reactions cannot be reliably predicted by any identified demographic or clinical characteristics. During the first 3 months of treatment, patients receiving ticlopidine must, therefore, be hematologically and clinically monitored for evidence of neutropenia or TTP. If any such evidence is seen, ticlopidine should be immediately discontinued. |
Conidition 1
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
(Description)
Labor and Delivery
(Description)
Nursing Mothers
(Description)
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Solution
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
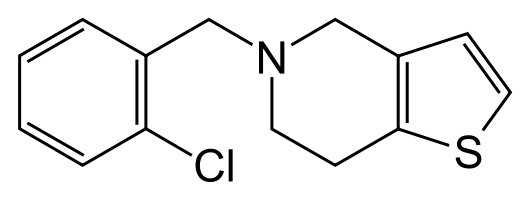
| |
Ticlopidine
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Ticlopidine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Ticlopidine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ticlopidine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Ticlopidine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Ticlopidine Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.