Reye's syndrome
For patient information click here
| Reye's syndrome | |
| ICD-10 | G93.7 |
|---|---|
| ICD-9 | 331.81 |
| DiseasesDB | 11463 |
| MedlinePlus | 001565 |
|
WikiDoc Resources for Reye's syndrome |
|
Articles |
|---|
|
Most recent articles on Reye's syndrome Most cited articles on Reye's syndrome |
|
Media |
|
Powerpoint slides on Reye's syndrome |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Reye's syndrome at Clinical Trials.gov Trial results on Reye's syndrome Clinical Trials on Reye's syndrome at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Reye's syndrome NICE Guidance on Reye's syndrome
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Reye's syndrome Discussion groups on Reye's syndrome Patient Handouts on Reye's syndrome Directions to Hospitals Treating Reye's syndrome Risk calculators and risk factors for Reye's syndrome
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Reye's syndrome |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Reye's syndrome is a potentially fatal disease that causes numerous detrimental effects to many organs, especially the brain and liver. It is associated with aspirin consumption by children with viral diseases such as chicken pox.
Historical Perspective
The syndrome is named after Dr R. Douglas Reye, who, along with fellow Australians Dr. Graeme Morgan and Dr. Jim Baral, published the first study of the syndrome in 1963 in the British medical journal called The Lancet.[1] In retrospect, the occurrence of the syndrome may have first been reported in 1929. Also in 1963, Dr. George Johnson and colleagues published an investigation of an outbreak of influenza B that described 16 children who developed neurological problems, four of whom had a remarkably similar profile to Reye’s syndrome. Some investigators refer to this disorder as Reye-Johnson syndrome, although it is more commonly called Reye's syndrome. During the late 1970s and early 1980s, studies in Ohio, Michigan and Arizona[2]pointed to the use of aspirin during an upper respiratory tract or chicken pox infection as a possible trigger of the syndrome. Beginning in 1980, the CDC cautioned physicians and parents about the association between Reye’s syndrome and the use of salicylates in children and teenagers with chickenpox or virus-like illnesses. In 1982 the US Surgeon General issued an advisory and in 1986 the Food and Drug Administration required a Reye’s syndrome-related warning label for all aspirin-containing medications.
Pathophysiology
The disease causes fatty liver with minimal inflammation, and severe encephalopathy (with swelling of the brain). The liver may become slightly enlarged and firm, and there is a change in the appearance of the kidneys. Jaundice is not usually present.[3]
The precise mechanism by which Reye's syndrome occurs remains unknown. However, the major form of Reye’s syndrome reported in the United States is characteristically preceded by a viral-like flu illness or chickenpox. Many studies have demonstrated a strong association between aspirin taken for these viral illnesses and the development of Reye’s syndrome.
The serious symptoms of Reye's syndrome appear to result from damage to cellular mitochondria, at least in the liver, and there are a number of ways that aspirin could cause or exacerbate mitochondrial damage. An increased risk of contracting Reye's syndrome is one of the main reasons that aspirin has not been recommended for use in children and teenagers, the age group for which the risk of lasting serious effects is highest.
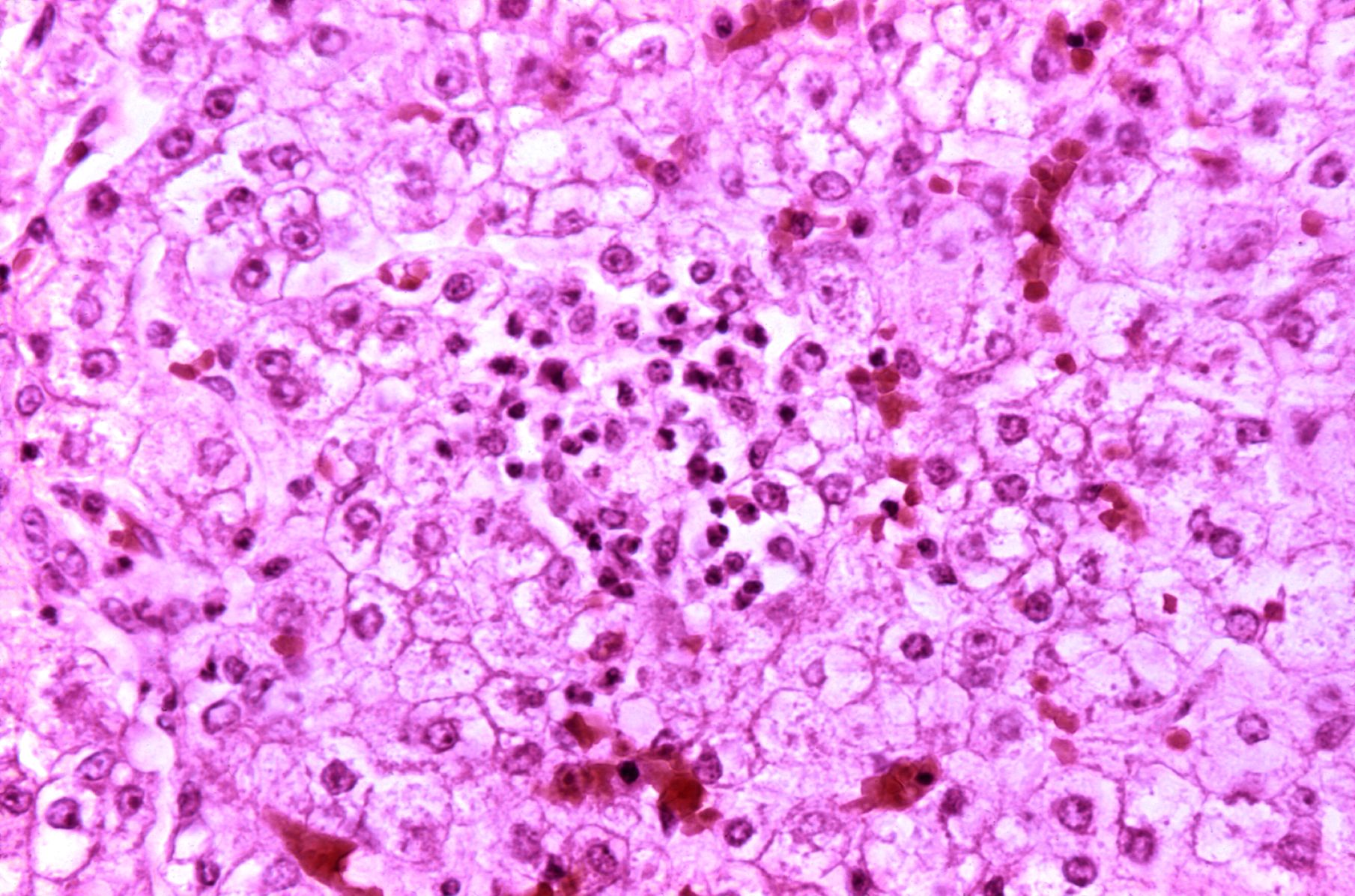
Microscopic Pathology
Diffuse panlobular steatosis and microvesicular fatty droplets with central nuclei are consistent with typical Reye's syndrome. Few patients may have multifocal spotty necrosis or centrilobular necrosis.
Differentiating Reye syndrome from other Diseases
Causes for similar symptoms include
- Various inborn metabolic disorders
- Viral encephalitis
- Drug overdose or poisoning
- Head trauma
- Hepatic failure due to other causes
- Meningitis
- Renal failure
Epidemiology and Demographics
Reye’s syndrome occurs almost exclusively in children although it has been reported to occur in adults. However, adults do not appear to be as vulnerable to permanent neural or liver damage. Unlike in the UK and Australia, the surveillance for Reye’s syndrome in the US is focused on patients under 18 years of age.
In 1980, after CDC began cautioning physicians and parents about the association between Reye’s syndrome and the use of salicylates in children with chickenpox or virus-like illnesses, the incidence of Reye's syndrome in the United States began to decline. In the United States between 1980 and 1997, the number of reported cases of Reye’s syndrome decreased from 555 cases in 1980 to about 2 cases per year since 1994. During this time period 93% of reported cases for which racial data were available occurred in whites and the median age was six years. A viral illness occurred in 93% of cases in the preceding three week period. For the period 1991-1994, the annual rate of hospitalizations due to Reye’s syndrome in the US was estimated to be between 0.2 and 1.1 per million population less than 18 years of age.
During the 1980s, a case-control study carried out in the United Kingdom also demonstrated an associate between Reye’s syndrome and aspirin exposure.[4] In June 1986, the United Kingdom Committee on Safety of Medicines issued warnings against the use of aspirin in children under 12 years of age and warning labels on aspirin-containing medications were introduced. UK surveillance for Reye’s syndrome documented a decline in the incidence of Reye’s syndrome following 1986. The reported incidence rate of Reye’s syndrome decreased from a high of 0.63 per 100,000 population less than 12 years of age in 1983/84 to 0.11 in 1990/91.
From November 1995 to November 1996 in France, a national survey of pediatric departments for children under 15 years of age with unexplained encephalopathy and a three fold (or greater) increase in serum aminotransferase and/or ammonia led to the identification of nine definite cases of Reye’s syndrome (0.79 cases per million children). Eight of the nine children with Reye’s syndrome were found to have been exposed to aspirin. In part because of this survey result, the French Medicines Agency reinforced the international attention to the relationship between aspirin and Reye’s syndrome by issuing its own public and professional warnings about this relationship.[5]
Some studies indicate that a significant percentage of cases, particularly in very young children, are later re-categorized as other disorders or conditions -- as high as 25% in the UK and 50% in Australia. These re-categorized disorders, unlike the characteristic Reye’s syndrome, are not strongly linked to exposure to aspirin.
Risk Factors
At least five epidemiologic studies published in US medical journals,[2] including one study that was supported by funds from the aspirin industry,[6] have confirmed an association between the development of Reye's syndrome and the use of aspirin (a salicylate compound) for treating the symptoms of influenza-like illnesses or chicken pox.[2] The Centers for Disease Control and Prevention (CDC), the U.S. Surgeon General, the American Academy of Pediatrics (AAP) and the Food and Drug Administration (FDA) recommend that aspirin and combination products containing aspirin not be given to children under 19 years of age during episodes of fever-causing illnesses. Acetylsalicylate is another word for aspirin; some medicine labels may use the words acetylsalicylate or acetylsalicylic acid instead of the word aspirin. Investigators at CDC have also cautioned against the use of medicines, including some anti-nausea medications that contain salicylic acid, or salicylate. A doctor or pharmacist should be consulted before any child or teenager under 19 years of age is given any medication containing aspirin (acetylsalicylate, salicylate, acetylsalicylic acid, or salicylic acid).
The vast majority of children who take aspirin while ill with a virus such as chicken pox or flu do not develop Reye's syndrome and some children who are diagnosed with the syndrome may not have taken aspirin or salicylates. Misdiagnoses of metabolic disorders that present with Reye’s Syndrome-like signs and symptoms, unreported exposures to aspirin, and possible other causes of the syndrome often account for these patients diagnosed with Reye’s syndrome but apparently unexposed to salicylates.
Natural History, Complications and Prognosis
Reye's syndrome progresses through five stages, explained below:
- Stage I
- Persistent, heavy vomiting that is not relieved by eating
- Generalized lethargy
- General mental symptoms, e.g. confusion
- Nightmares
- Stage II
- Stupor caused by minor brain inflammation
- Hyperventilation
- Fatty liver (found by biopsy)
- Hyperactive reflexes
- Stage III
- Continuation of Stage I and II symptoms
- Possible coma
- Possible cerebral edema
- Rarely, respiratory arrest
- Stage IV
- Deepening coma
- Large pupils with minimal response to light
- Minimal but still present hepatic dysfunction
- Stage V
- Very rapid onset following stage IV
- Deep coma
- Seizures
- Respiratory failure
- Flaccidity
- Extremely high blood ammonia (above 300mg per 100mL of blood)
- Death
Prognosis
Documented cases of Reye’s syndrome in adults have only been very rarely reported. The recovery of adults with the syndrome is generally complete, with liver and brain function returning to normal within two weeks of the illness. In children however, mild to severe permanent brain damage is possible, especially in infants. Over thirty percent of the cases reported in the United States from 1981 through 1997 died. Early diagnosis is vital, otherwise death or severe brain damage may follow.
References
- ↑ Reye RD, Morgan G, Baral J (1963). "Encephalopathy and fatty degeneration of the viscera. A Disease entity in childhood". Lancet. 2: 749–52. PMID 14055046.
- ↑ 2.0 2.1 2.2 Mortimer EA (1987). "Reye's syndrome, salicylates, epidemiology, and public health policy". JAMA. 257 (14): 941. PMID 3820516.
- ↑ Suchy, FJ, el al.; Sokol, RJ; Balistreri, WF (2007). Liver Disease in Children. Cambridge: Cambridge University Press. ISBN 0-5218-5657-4.
- ↑ Hall SM, Plaster PA, Glasgow JF, Hancock P (1988). "Preadmission antipyretics in Reye's syndrome". Arch. Dis. Child. 63 (7): 857–66. PMID 3415311.
- ↑ Autret-Leca E, Jonville-Béra AP, Llau ME; et al. (2001). "Incidence of Reye's syndrome in France: a hospital-based survey". Journal of clinical epidemiology. 54 (8): 857–62. PMID 11470397.
- ↑ Forsyth BW, Horwitz RI, Acampora D; et al. (1989). "New epidemiologic evidence confirming that bias does not explain the aspirin/Reye's syndrome association". JAMA. 261 (17): 2517–24. PMID 2704111.
External links
Template:CNS diseases of the nervous system