Pulmonary alveolar proteinosis
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Mahda Alihashemi M.D. [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17]
Synonyms and keywords:Pulmonary alveolar phospholipoproteinosis ; Synonym 2; Synonym 3
Overview
Historical Perspective
- Pulmonary alveolar proteinosis ( PAP) was first discovered by Samuel Rosen, Benjamin Castleman, and Averill Liebow, in 1958 during pathologic investigations of material filling the alveoli. [1]
- In [year], [gene] mutations were first identified in the pathogenesis of [disease name].
- In 1960, the first therapeutic bronchoalveolar lavage by repeated segmental flooding was developed by Dr. Jose Ramirez-Rivera to treat pulmonary alveolar proteinosis. [2]
Classification
Pulmonary alveolar proteinosis ( PAP) may be classified into 2 subtypes:
Primary Pulmonary alveolar proteinosis:
- Autoimmune and hereditary PAP: Disruption of Granulocyte-macrophage colony-stimulating factor (GM-CSF) signalling[3]
- Clearance of surfactant by alveolar macrophages is regulated by GM-CSF
- Autoimmune PAP: The most common type of PAP in adults
- Effect of GM-CSF on alveolar macrophages is neutralized by antibodies to GM-CSF
- Hereditary PAP :GM-CSF is intact but recessive variants of the GM-CSF receptor alpha and beta genes (CSF2RA and CSF2RB) impair signaling by GM-CSF
- Congenital PAP: Disorders of surfactant production
- Autoimmune and hereditary PAP: Disruption of Granulocyte-macrophage colony-stimulating factor (GM-CSF) signalling[3]
Secondary pulmonary alveolar proteinosis:
Pathophysiology
- The pathogenesis of pulmonary alveolar proteinosis is characterized by the intraalveolar accumulation of surfactant phospholipid and apoproteins. Pulmonary alveolar proteinosis develop because of reduced granulocyte-macrophage colony-stimulating factor (GM-CSF) levels or function and/or impaired alveolar macrophage function.[12]
- Genes involved in the pathogenesis of pulmonary alveolar proteinosis include:
- Surfactant proteins B and C (SFTPB, SFTPC) , ATP-binding cassette, subfamily A (ABCA3) , SLC7A7, MARS (Methionyl-tRNA synthetasegene ) and GM-CSF receptor alpha and beta genes (CSF2RA and CSF2RB) genes mutation in production of surfactant
- On gross pathology after lung wasings, fluid with milky composition are characteristic finding of pulmonary alveolar proteinosis. [13]
- On microscopic histopathological analysis, terminal bronchioles and alveoli are filled with a lipoproteinaceous material that will be pink after periodic acid-Schiff (PAS) stain. Oil red-O positive, PAS-positive macrophages and cholesterol crystals are another characteristic findings of pulmonary alveolar proteinosis. Multilamellated structures in the alveoli can be seen in electron microscopy.[14]
Causes
The exact etiology of pulmonary alveolar proteinosis (PAP) is unknown, but it may be associated with:
- Exposure to insecticides, titanium dioxide, indium-tin oxide and silica dust[9]
- Hematologic malignancies[10]
- HIV infection (AIDS)[15]
- Leflunomide, case report[16]
- Recessive CSF2RA mutations
Differentiating [disease name] from other Diseases
- [Disease name] must be differentiated from other diseases that cause [clinical feature 1], [clinical feature 2], and [clinical feature 3], such as:
- [Differential dx1]
- [Differential dx2]
- [Differential dx3]
Epidemiology and Demographics
- The prevalence of pulmonary alveolar proteinosis is approximately 1 per 100,000 individuals worldwide.
- The incidence of pulmonary alveolar proteinosis is estimated to be 0.33 cases per 100,000 individuals in united states. [17]
Age
- Pulmonary alveolar proteinosis is more commonly observed among patients aged 40- 50 years old.[18]
Gender
- Men are more commonly affected with pulmonary alveolar proteinosis than women. The men to women ratio is approximately 2 to 1.
Race
- There is no racial predilection for pulmonary alveolar protoeinosis.
Risk Factors
- Common risk factors in the development of pulmonary alveolar proteinosis are
Natural History, Complications and Prognosis
- If left untreated, patients with pulmonary alveolar proteinosis may progress to develop pulmonary fibrosis, cor pulmonale. The natural history of secondary pulmonary alveolar proteinosis is related to underlying etiologies
- Common complications of pulmonary alveolar proteinosis include lung infections with Pneumocystis carinii, Mycobacterium avium-intracellulare , N asteroides , [20]
- Prognosis is generally good for primary pulmonary alveolar proteinosis with whole lung lavage. Congenital pulmonary alveolar proteinosis respond well to lung transplantation. Prognosis for secondary pulmonary alveolar proteinosis is related to underlying etiologies.
Diagnosis
Diagnostic Criteria
- The diagnosis of [disease name] is made when at least [number] of the following [number] diagnostic criteria are met:
- [criterion 1]
- [criterion 2]
- [criterion 3]
- [criterion 4]
Symptoms
- One third of patients are usually asymptomatic.[21]
- Symptoms of pulmonary alveolar proteinosis may include the following:[22]
- Dyspnea on exertion
- Cough ( productive or nonproduvtive)
- Sputum
- Fatigue
- Weight loss
- Low-grade fever
Physical Examination
- The physical examination is often normal. [23]
- Patients with [disease name] usually appear [general appearance].
- Physical examination may be remarkable for:
Laboratory Findings
- Serum anti-granulocyte-macrophage colony-stimulating factor (GM-CSF) antibodies in autoimmune pulmonary alveolar proteinosis[26]
- Complete blood count and differential in secondary pulmonary alveolar proteinosis due to hematologic malignancy.
- Elevated serum level of GM-CSF in hereditary pulmonary alveolar proteinosis[27]
- Nonspecific laboratory findings:[28]
- Hypergammaglobulinemia
- Polycythemia
- Increased lactate dehydrogenase (LDH) levels
- Elevated levels of lung surfactant proteins A and D (SP-A and SP-D)
- Elevated levels of tumor markers (carcinoembryonic antigen [CEA], sialyl SSEA-1 [SLX], carbohydrate antigens sialyl Lewis-a [CA 19-9]) [29]
Imaging Findings
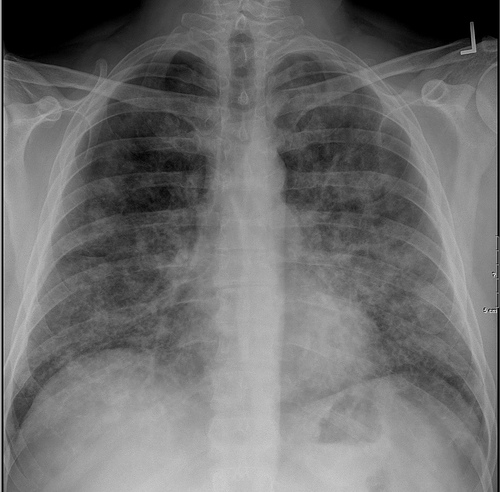
Chest X-ray:
- Bat wing distribution of bilateral symmetric alveolar opacities in mid and lower lung zones [30]
- Segmental atelectasis due to bronchiolar obstruction [31]
- In chronic cases, focal fibrosis may be seen[32]
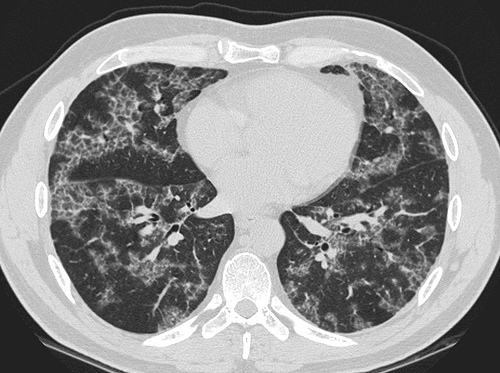
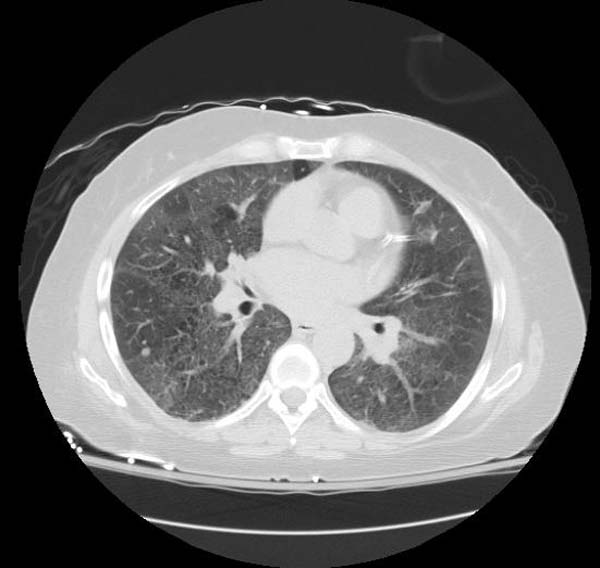
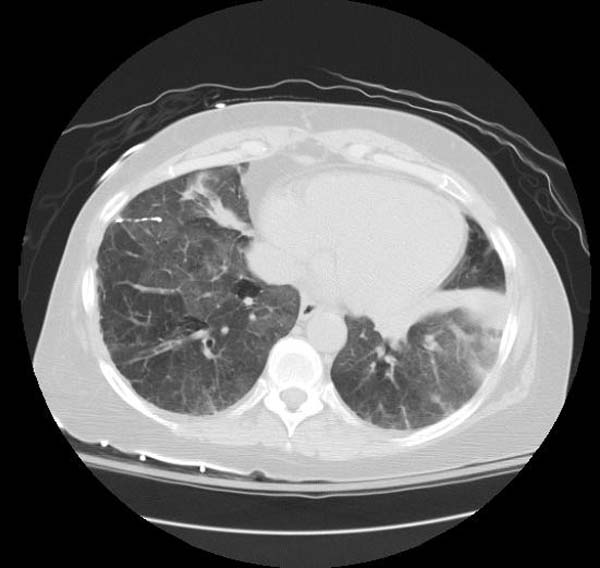
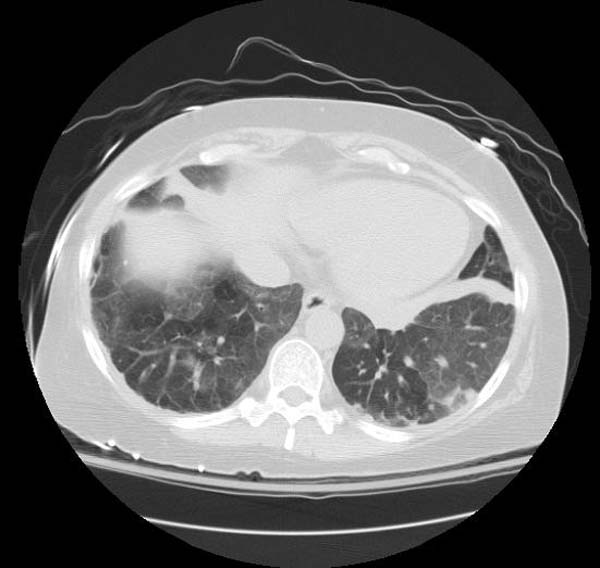
High resolution computed tomography (HRCT):
- Ground-glass opacification with crazy-paving pattern ( interlobular septal thickening )[33]
HRCT typically reveals ground-glass opacification, predominantly in a homogeneous distribution. There may also be thickened intralobular structures and interlobular septa in typical polygonal shapes superimposed on ground glass opacities, referred to as "crazy-paving" (table 2 and image 2) [65,84]. In a series of 42 patients, a patchy, geographic pattern with crazy-paving was common among patients with autoimmune PAP, whereas a pattern of diffuse ground glass opacity without crazy-paving was more common among patients with secondary PAP [84].
However, crazy-paving is not specific for PAP and can also be observed in patients with the acute respiratory distress syndrome, organizing pneumonia, lipoid pneumonia, acute interstitial pneumonia/diffuse alveolar damage (AIP/DAD), drug-related hypersensitivity reactions, and AIP/DAD superimposed on usual interstitial pneumonitis [85,86]. (See "High resolution computed tomography of the lungs", section on 'Ground glass opacification'.)
- There are no [imaging study] findings associated with [disease name].
- [Imaging study 1] is the imaging modality of choice for [disease name].
- On [imaging study 1], [disease name] is characterized by [finding 1], [finding 2], and [finding 3].
- [Imaging study 2] may demonstrate [finding 1], [finding 2], and [finding 3].
Other Diagnostic Studies
Pulmonary function test:
- Reduction in the diffusing capacity for carbon monoxide (DLCO) [34]
- Maybe decrease in forced vital capacity[35]
- [Disease name] may also be diagnosed using [diagnostic study name].
- Findings on [diagnostic study name] include [finding 1], [finding 2], and [finding 3].
Treatment
Medical Therapy
- There is no treatment for [disease name]; the mainstay of therapy is supportive care.
- The mainstay of therapy for [disease name] is [medical therapy 1] and [medical therapy 2].
- [Medical therapy 1] acts by [mechanism of action 1].
- Response to [medical therapy 1] can be monitored with [test/physical finding/imaging] every [frequency/duration].
Surgery
- Surgery is the mainstay of therapy for [disease name].
- [Surgical procedure] in conjunction with [chemotherapy/radiation] is the most common approach to the treatment of [disease name].
- [Surgical procedure] can only be performed for patients with [disease stage] [disease name].
Prevention
- There are no primary preventive measures available for [disease name].
- Effective measures for the secondary prevention of pulmonary alveolar proteinosis due to inorganic dust or insecticides should avoid further exposure.
- Once diagnosed and successfully treated, patients with [disease name] are followed-up every [duration]. Follow-up testing includes [test 1], [test 2], and [test 3].
References
- ↑ Rosen, Samuel H.; Castleman, Benjamin; Liebow, Averill A.; Enzinger, Frank M.; Hunt, Richard T. N. (1958). "Pulmonary Alveolar Proteinosis". New England Journal of Medicine. 258 (23): 1123–1142. doi:10.1056/NEJM195806052582301. ISSN 0028-4793.
- ↑ Ramirez-R., Jose; Nyka, Walenty; McLaughlin, Joseph (1963). "Pulmonary Alveolar Proteinosis". New England Journal of Medicine. 268 (4): 165–171. doi:10.1056/NEJM196301242680401. ISSN 0028-4793.
- ↑ Suzuki T, Trapnell BC (September 2016). "Pulmonary Alveolar Proteinosis Syndrome". Clin. Chest Med. 37 (3): 431–40. doi:10.1016/j.ccm.2016.04.006. PMID 27514590.
- ↑ Brasch F, Birzele J, Ochs M, Guttentag SH, Schoch OD, Boehler A, Beers MF, Müller KM, Hawgood S, Johnen G (September 2004). "Surfactant proteins in pulmonary alveolar proteinosis in adults". Eur. Respir. J. 24 (3): 426–35. doi:10.1183/09031936.04.00076403. PMID 15358702.
- ↑ Mulugeta S, Gray JM, Notarfrancesco KL, Gonzales LW, Koval M, Feinstein SI, Ballard PL, Fisher AB, Shuman H (June 2002). "Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3". J. Biol. Chem. 277 (25): 22147–55. doi:10.1074/jbc.M201812200. PMID 11940594.
- ↑ Salerno T, Peca D, Menchini L, Schiavino A, Petreschi F, Occasi F, Cogo P, Danhaive O, Cutrera R (March 2014). "Respiratory insufficiency in a newborn with congenital hypothyroidism due to a new mutation of TTF-1/NKX2.1 gene". Pediatr. Pulmonol. 49 (3): E42–4. doi:10.1002/ppul.22788. PMID 23997037.
- ↑ "GeneReviews® - NCBI Bookshelf".
- ↑ Hadchouel A, Wieland T, Griese M, Baruffini E, Lorenz-Depiereux B, Enaud L, Graf E, Dubus JC, Halioui-Louhaichi S, Coulomb A, Delacourt C, Eckstein G, Zarbock R, Schwarzmayr T, Cartault F, Meitinger T, Lodi T, de Blic J, Strom TM (May 2015). "Biallelic Mutations of Methionyl-tRNA Synthetase Cause a Specific Type of Pulmonary Alveolar Proteinosis Prevalent on Réunion Island". Am. J. Hum. Genet. 96 (5): 826–31. doi:10.1016/j.ajhg.2015.03.010. PMC 4570277. PMID 25913036.
- ↑ 9.0 9.1 Buechner HA, Ansari A (April 1969). "Acute silico-proteinosis. A new pathologic variant of acute silicosis in sandblasters, characterized by histologic features resembling alveolar proteinosis". Dis Chest. 55 (4): 274–8. PMID 5775743.
- ↑ 10.0 10.1 Cordonnier C, Fleury-Feith J, Escudier E, Atassi K, Bernaudin JF (March 1994). "Secondary alveolar proteinosis is a reversible cause of respiratory failure in leukemic patients". Am. J. Respir. Crit. Care Med. 149 (3 Pt 1): 788–94. doi:10.1164/ajrccm.149.3.8118651. PMID 8118651.
- ↑ Sharma S, Nadrous HF, Peters SG, Tefferi A, Litzow MR, Aubry MC, Afessa B (September 2005). "Pulmonary complications in adult blood and marrow transplant recipients: autopsy findings". Chest. 128 (3): 1385–92. doi:10.1378/chest.128.3.1385. PMID 16162733.
- ↑ Carey B, Trapnell BC (May 2010). "The molecular basis of pulmonary alveolar proteinosis". Clin. Immunol. 135 (2): 223–35. doi:10.1016/j.clim.2010.02.017. PMC 2866141. PMID 20338813.
- ↑ Mikami T, Yamamoto Y, Yokoyama M, Okayasu I (December 1997). "Pulmonary alveolar proteinosis: diagnosis using routinely processed smears of bronchoalveolar lavage fluid". J. Clin. Pathol. 50 (12): 981–4. PMC 500376. PMID 9516877.
- ↑ Maygarden SJ, Iacocca MV, Funkhouser WK, Novotny DB (June 2001). "Pulmonary alveolar proteinosis: a spectrum of cytologic, histochemical, and ultrastructural findings in bronchoalveolar lavage fluid". Diagn. Cytopathol. 24 (6): 389–95. PMID 11391819.
- ↑ 15.0 15.1 Juvet SC, Hwang D, Waddell TK, Downey GP (2008). "Rare lung disease II: pulmonary alveolar proteinosis". Can. Respir. J. 15 (4): 203–10. PMC 2677953. PMID 18551202.
- ↑ Wardwell NR, Miller R, Ware LB (September 2006). "Pulmonary alveolar proteinosis associated with a disease-modifying antirheumatoid arthritis drug". Respirology. 11 (5): 663–5. doi:10.1111/j.1440-1843.2006.00905.x. PMID 16916345.
- ↑ Hunt S, Miller AL, Schissel S, Ross JJ (December 2010). "A crazy cause of dyspnea". N. Engl. J. Med. 363 (25): e38. doi:10.1056/NEJMimc1008281. PMID 21158654.
- ↑ Inoue Y, Trapnell BC, Tazawa R, Arai T, Takada T, Hizawa N, Kasahara Y, Tatsumi K, Hojo M, Ichiwata T, Tanaka N, Yamaguchi E, Eda R, Oishi K, Tsuchihashi Y, Kaneko C, Nukiwa T, Sakatani M, Krischer JP, Nakata K (April 2008). "Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan". Am. J. Respir. Crit. Care Med. 177 (7): 752–62. doi:10.1164/rccm.200708-1271OC. PMC 2720118. PMID 18202348.
- ↑ Suzuki T, Trapnell BC (September 2016). "Pulmonary Alveolar Proteinosis Syndrome". Clin. Chest Med. 37 (3): 431–40. doi:10.1016/j.ccm.2016.04.006. PMID 27514590.
- ↑ Abdul Rahman JA, Moodley YP, Phillips MJ (August 2004). "Pulmonary alveolar proteinosis associated with psoriasis and complicated by mycobacterial infection: successful treatment with granulocyte-macrophage colony stimulating factor after a partial response to whole lung lavage". Respirology. 9 (3): 419–22. PMID 15497254.
- ↑ Inoue Y, Trapnell BC, Tazawa R, Arai T, Takada T, Hizawa N, Kasahara Y, Tatsumi K, Hojo M, Ichiwata T, Tanaka N, Yamaguchi E, Eda R, Oishi K, Tsuchihashi Y, Kaneko C, Nukiwa T, Sakatani M, Krischer JP, Nakata K (April 2008). "Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan". Am. J. Respir. Crit. Care Med. 177 (7): 752–62. doi:10.1164/rccm.200708-1271OC. PMC 2720118. PMID 18202348.
- ↑ Suzuki T, Trapnell BC (September 2016). "Pulmonary Alveolar Proteinosis Syndrome". Clin. Chest Med. 37 (3): 431–40. doi:10.1016/j.ccm.2016.04.006. PMID 27514590.
- ↑ Suzuki T, Trapnell BC (September 2016). "Pulmonary Alveolar Proteinosis Syndrome". Clin. Chest Med. 37 (3): 431–40. doi:10.1016/j.ccm.2016.04.006. PMID 27514590.
- ↑ Goldstein LS, Kavuru MS, Curtis-McCarthy P, Christie HA, Farver C, Stoller JK (November 1998). "Pulmonary alveolar proteinosis: clinical features and outcomes". Chest. 114 (5): 1357–62. PMID 9824014.
- ↑ Inoue Y, Trapnell BC, Tazawa R, Arai T, Takada T, Hizawa N, Kasahara Y, Tatsumi K, Hojo M, Ichiwata T, Tanaka N, Yamaguchi E, Eda R, Oishi K, Tsuchihashi Y, Kaneko C, Nukiwa T, Sakatani M, Krischer JP, Nakata K (April 2008). "Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan". Am. J. Respir. Crit. Care Med. 177 (7): 752–62. doi:10.1164/rccm.200708-1271OC. PMC 2720118. PMID 18202348.
- ↑ Suzuki T, Trapnell BC (September 2016). "Pulmonary Alveolar Proteinosis Syndrome". Clin. Chest Med. 37 (3): 431–40. doi:10.1016/j.ccm.2016.04.006. PMID 27514590.
- ↑ Suzuki T, Trapnell BC (September 2016). "Pulmonary Alveolar Proteinosis Syndrome". Clin. Chest Med. 37 (3): 431–40. doi:10.1016/j.ccm.2016.04.006. PMID 27514590.
- ↑ Martin RJ, Rogers RM, Myers NM (June 1978). "PUlmonary alveolar proteinosis: shunt fraction and lactic acid dehydrogenase concentration as aids to diagnosis". Am. Rev. Respir. Dis. 117 (6): 1059–62. doi:10.1164/arrd.1978.117.6.1059. PMID 666104.
- ↑ Suzuki T, Trapnell BC (September 2016). "Pulmonary Alveolar Proteinosis Syndrome". Clin. Chest Med. 37 (3): 431–40. doi:10.1016/j.ccm.2016.04.006. PMID 27514590.
- ↑ Patel SM, Sekiguchi H, Reynolds JP, Krowka MJ (2012). "Pulmonary alveolar proteinosis". Can. Respir. J. 19 (4): 243–5. PMC 3411387. PMID 22891182.
- ↑ Prakash UB, Barham SS, Carpenter HA, Dines DE, Marsh HM (June 1987). "Pulmonary alveolar phospholipoproteinosis: experience with 34 cases and a review". Mayo Clin. Proc. 62 (6): 499–518. PMID 3553760.
- ↑ Hudson AR, Halprin GM, Miller JA, Kilburn KH (June 1974). "Pulmonary interstitial fibrosis following alveolar proteinosis". Chest. 65 (6): 700–2. PMID 4832278.
- ↑ Patel SM, Sekiguchi H, Reynolds JP, Krowka MJ (2012). "Pulmonary alveolar proteinosis". Can. Respir. J. 19 (4): 243–5. PMC 3411387. PMID 22891182.
- ↑ Inoue Y, Trapnell BC, Tazawa R, Arai T, Takada T, Hizawa N, Kasahara Y, Tatsumi K, Hojo M, Ichiwata T, Tanaka N, Yamaguchi E, Eda R, Oishi K, Tsuchihashi Y, Kaneko C, Nukiwa T, Sakatani M, Krischer JP, Nakata K (April 2008). "Characteristics of a large cohort of patients with autoimmune pulmonary alveolar proteinosis in Japan". Am. J. Respir. Crit. Care Med. 177 (7): 752–62. doi:10.1164/rccm.200708-1271OC. PMC 2720118. PMID 18202348.
- ↑ Seymour JF, Presneill JJ (July 2002). "Pulmonary alveolar proteinosis: progress in the first 44 years". Am. J. Respir. Crit. Care Med. 166 (2): 215–35. doi:10.1164/rccm.2109105. PMID 12119235.
Overview
Pulmonary alveolar proteinosis -(PAP) is a rare lung disease in which abnormal accumulation of surfactant occurs within the alveoli, interfering with gas exchange. PAP can occur in a primary form or secondarily in the settings of malignancy (especially in myeloid leukemia), pulmonary infection, or environmental exposure to dusts or chemicals. Rare familial forms have also been recognized, suggesting a genetic component in some cases. [1] [2] [3] [4]
Historical Perspective
Pathophysiology
Although the cause of PAP remains obscure, a major breakthrough in the understanding of the etiology of the disease came by the chance observation that mice bred for experimental study to lack a hematologic growth factor known as granulocyte-macrophage colony stimulating factor (GM-CSF) developed a pulmonary syndrome of abnormal surfactant accumulation resembling human PAP. The implications of this finding are still being explored, but significant progress was reported in February, 2007. Researchers in that report discussed the presence of anti-GM-CSF autoantibodies in patients with PAP, and duplicated that syndrome with the infusion of these autoantibodies into mice. [5]
Causes
Pulmonary alveolar proteinosis is an idiopathic disease, but studies have linked causes to production of antibodies that neutralize GM-CSF, granulocyte-macrophage colony-stimulating factor. Occasionally, development of pulmonary alveolar proteinosis is related to exposure of toxic substances, such as inorganic dusts, infection with Pneumocystis jirovecii, certain cancers, and immunosuppressants. It rarely occurs in newborns.
There are several types of clinical forms of Pulmonary alveolar proteinosis; primary, secondary, and congenital. Primary PAP involves high levels of antibodies that neutralize GM-CSF, granulocyte-macrophage colony-stimulating factor. Secondary PAP occurs from clinical conditions that reduce the numbers and functions of alveolar macrophages. Congenital PAP is due to genetic mutations in the genes encoding surfactant proteins of the receptor for granulocyte-macrophage colony-stimulating factor.[6]
Causes
Common Causes
- Pneumocystis jirovecii
- Respiratory failure
- Uncontrolled infection
- Respiratory Pathogens
- Opportunistic Pathogens: Nocardia
- Sirolimus
- Prednisone
- Inorganic dusts
- Inhalation of silica dust
- Exposure to insecticides
- aluminum dust
- titanium dioxide
- Haematological malignancies
- HIV infection.
Causes by Organ System
| Cardiovascular | No underlying causes |
| Chemical / poisoning | No underlying causes |
| Dermatologic | No underlying causes |
| Drug Side Effect | Sirolimus, Prednisone |
| Ear Nose Throat | No underlying causes |
| Endocrine | No underlying causes |
| Environmental | Inorganic dusts, Inhalation of silica dust, Exposure to insecticides, aluminum dust, titanium dioxide |
| Gastroenterologic | No underlying causes |
| Genetic | No underlying causes |
| Hematologic | Haematological malignancies |
| Iatrogenic | No underlying causes |
| Infectious Disease | Uncontrolled infection, Respiratory Pathogens, Nocardia, HIV infection |
| Musculoskeletal / Ortho | [No underlying causes |
| Neurologic | No underlying causes |
| Nutritional / Metabolic | No underlying causes |
| Obstetric/Gynecologic | No underlying causes |
| Oncologic | Haematological malignancies |
| Opthalmologic | No underlying causes |
| Overdose / Toxicity | No underlying causes |
| Psychiatric | No underlying causes |
| Pulmonary | Pneumocystis jirovecii, Respiratory failure |
| Renal / Electrolyte | No underlying causes |
| Rheum / Immune / Allergy | No underlying causes |
| Sexual | No underlying causes |
| Trauma | No underlying causes |
| Urologic | No underlying causes |
| Dental | No underlying causes |
| Miscellaneous | No underlying causes |
Causes in Alphabetical Order
|
Natural History
The clinical course of PAP is unpredictable. Spontaneous remission is recognized; some patients have stable symptoms.
Complications
Death may occur due to progression of PAP or due to the underlying disease associated with PAP. Individuals with PAP are more vulnerable to infection of the lung by bacteria or fungi.
Prognosis
History and Symptoms
The symptoms of PAP include:
Chest X Ray
Classic radiographic finding is bilateral, symmetric alveolar consolidation or ground-glass opacity, particularly in a perihilar or hilar distribution resembling pulmonary edema.
CT
- CT typically shows diffuse ground-glass attenuation with superimposed crazy-paving pattern (intra- and interlobular septal thickening, often in polygonal shapes representing the secondary pulmonary lobule).
Patient#1
Patient #1: Proven PAP but not quite the classic crazy-paving pattern
Other Diagnostic Studies
Diagnosis is generally made by surgical or endoscopic biopsy of the lung, revealing the distinctive pathologic finding.
Histopathological Findings: Pulmonary alveolar proteinosis
{{#ev:youtube|L9fVRzcPkEQ}}
Medical Therapy
The use of GM-CSF injections has also been attempted, with variable success.
Surgery
The standard treatment for PAP is whole-lung lavage, in which sterile fluid is instilled into the lung and then removed, along with the abnormal surfactant material. This is generally effective at ameliorating symptoms, often for prolonged periods. Lung transplantation can be performed in refractory cases.
References
- ↑ Rosen SH, Castleman B, and Liebow AA. Pulmonary alveolar proteinosis. New England Journal of Medicine 1958; 258: 1123-1142.
- ↑ Seymour JF and Presneill JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. American Journal of Respiratory and Critical Care Medicine 2002; 166: 215-235.
- ↑ Shah PL, Hansell D, Lawson PR, et al. Pulmonary alveolar proteinosis; clinical aspects and current concepts on pathogenesis. Thorax 2000; 55: 67-77.
- ↑ Stanley E, Lieschke GJ, Grail D, et al. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc. Natl. Acad. Sci. USA 1994; 91: 5592-5596.
- ↑ Uchida K, Beck D, Yamamoto T, Berclaz P, Abe S, Staudt M, Carey B, Filippi M, Wert S, Denson L, Puchalski J, Hauck D, Trapnell B (2007). "GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis". N Engl J Med. 356 (6): 567–79. PMID 17287477.
- ↑ 6.0 6.1 Trapnell BC, Whitsett JA, Nakata K (2003). "Pulmonary alveolar proteinosis". N Engl J Med. 349 (26): 2527–39. doi:10.1056/NEJMra023226. PMID 14695413.