Propofol: Difference between revisions
No edit summary |
No edit summary |
||
| (9 intermediate revisions by the same user not shown) | |||
| Line 4: | Line 4: | ||
|aOrAn=a | |aOrAn=a | ||
|drugClass=general anesthetic | |drugClass=general anesthetic | ||
|indication=general anesthesia,monitored anesthesia care sedation, sedation for a mechanically ventilated patient, intensive care unit | |indication=general anesthesia, monitored anesthesia care sedation, sedation for a mechanically ventilated patient, intensive care unit | ||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions=[[dermatologic]]: injection site pain (up to 28.5% ), gastrointestinal: nausea and vomiting (2% to 2.5% ), musculoskeletal: involuntary movement, muscle (17%) | |adverseReactions=[[dermatologic]]: injection site pain (up to 28.5% ), [[gastrointestinal]]: [[nausea]] and [[vomiting]] (2% to 2.5% ), [[musculoskeletal]]: [[involuntary movement]], muscle (17%) | ||
|blackBoxWarningTitle=<b><span style="color:#FF0000;"> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">For Intravenous Administration</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;"></span></i> Rx only | |blackBoxWarningBody=<i><span style="color:#FF0000;"></span></i> Rx only | ||
* Strict aseptic technique must always be maintained during handling. Propofol Injectable Emulsion is a single access parenteral product (single patient infusion vial) which contains 0.005% disodium edetate (EDTA) to inhibit the rate of growth of microorganisms, for up to 12 hours, in the event of accidental extrinsic contamination. However, Propofol Injectable Emulsion can still support the growth of microorganisms, as it is not an antimicrobially preserved product under USP standards. Do not use if contamination is suspected. Discard unused drug product as directed within the required time limits. There have been reports in which failure to use aseptic technique when handling Propofol Injectable Emulsion was associated with microbial contamination of the product and with fever, infection/sepsis, other life-threatening illness, and/or death. | |||
Strict aseptic technique must always be maintained during handling. | * There have been reports, in the literature and other public sources, of the transmission of bloodborne pathogens (such as Hepatitis B, Hepatitis C, and HIV) from unsafe injection practices, and use of propofol vials intended for single use on multiple persons. Propofol Injectable Emulsion vials are never to be accessed more than once or used on more than one person. | ||
(See Warnings and Dosage and Administration, Handling Procedures). | |||
There have been reports, in the literature and other public sources, of the transmission of bloodborne pathogens (such as Hepatitis B, Hepatitis C, and HIV) from unsafe injection practices, and use of propofol vials intended for single use on multiple persons. | |fdaLIADAdult= | ||
* General anesthesia: (healthy adults less than 55 years of age) induction, 40 mg IV every 10 seconds until induction onset (2 to 2.5 mg/kg); dose varies for age and surgery type. | |||
(See | * General anesthesia: (healthy adults less than 55 years of age) maintenance, 100 to 200 mcg/kg/min IV infusion (6 to 12 mg/kg/hr); dose varies for age and surgery type. | ||
|fdaLIADAdult=General anesthesia: (healthy adults less than 55 years of age) induction, 40 mg IV every 10 seconds until induction onset (2 to 2.5 mg/kg); dose varies for age and surgery type | * General anesthesia: (healthy adults less than 55 years of age) maintenance, 20 to 50 mg increments IV bolus as needed. | ||
General anesthesia: (healthy adults less than 55 years of age) maintenance, 100 to 200 mcg/kg/min IV infusion (6 to 12 mg/kg/hr); dose varies for age and surgery type | * Migraine: 10 mg slow IV push, every 5 to 10 minutes, max 80 mg; mixed with 1 mL lidocaine 2% per 10 mL of propofol 1%; infuse at rate of 1 mL/10 sec. | ||
General anesthesia: (healthy adults less than 55 years of age) maintenance, 20 to 50 mg increments IV bolus as needed | * Monitored anesthesia care sedation: (healthy adults less than 55 years of age) MAC initiation: 100 to 150 mcg/kg/min (6 to 9 mg/kg/hr) IV infusion or 0.5 mg/kg slow IV injection for 3 to 5 min followed immediately by maintenance infusion. | ||
Migraine: 10 mg slow IV push, every 5 to 10 minutes, | * Monitored anesthesia care sedation: (healthy adults less than 55 years of age) MAC maintenance: 25 to 75 mcg/kg/min (1.5 to 4.5 mg/kg/hr) IV infusion or 10 to 20 mg incremental IV bolus doses. | ||
Monitored anesthesia care sedation: (healthy adults less than 55 years of age) MAC initiation: 100 to 150 mcg/kg/min (6 to 9 mg/kg/hr) IV infusion or 0.5 mg/kg slow IV injection for 3 to 5 min followed immediately by maintenance infusion | * Procedural sedation: 1 mg/kg IV followed by 0.5 mg/kg every 3 to 5 minutes as needed for sedation. | ||
Monitored anesthesia care sedation: (healthy adults less than 55 years of age) MAC maintenance: 25 to 75 mcg/kg/min (1.5 to 4.5 mg/kg/hr) IV infusion or 10 to 20 mg incremental IV bolus doses | * Sedation for a mechanically ventilated patient, Intensive care unit: 5 mcg/kg/min (0.3 mg/kg/hr) IV infusion for 5 min then titrate in 5 to 10 mcg/kg/min (0.3 to 0.6 mg/kg/hr) increments to achieve desired level of sedation; allow minimum of 5 min between dose adjustments; usual maintenance rates 5 to 50 mcg/kg/min (0.3 to 3 mg/kg/hr) or higher. | ||
Procedural sedation: 1 mg/kg IV followed by 0.5 mg/kg every 3 to 5 minutes as needed for sedation | |||
Sedation for a mechanically ventilated patient, Intensive care unit: 5 mcg/kg/min (0.3 mg/kg/hr) IV infusion for 5 min then titrate in 5 to 10 mcg/kg/min (0.3 to 0.6 mg/kg/hr) increments to achieve desired level of sedation; allow minimum of 5 min between dose adjustments; usual maintenance rates 5 to 50 mcg/kg/min (0.3 to 3 mg/kg/hr) or higher | |||
|offLabelAdultGuideSupport=There is limited information about <i>Off-Label Guideline-Supported Use</i> of Propofol in adult patients. | |offLabelAdultGuideSupport=There is limited information about <i>Off-Label Guideline-Supported Use</i> of Propofol in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information about <i>Off-Label Non–Guideline-Supported Use</i> of Propofol in adult patients. | |offLabelAdultNoGuideSupport=There is limited information about <i>Off-Label Non–Guideline-Supported Use</i> of Propofol in adult patients. | ||
|offLabelPedGuideSupport=There is limited information about <i>Off-Label Guideline-Supported Use</i> of Propofol in pediatric patients. | |offLabelPedGuideSupport=There is limited information about <i>Off-Label Guideline-Supported Use</i> of Propofol in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information about <i>Off-Label Non–Guideline-Supported Use</i> of Propofol in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information about <i>Off-Label Non–Guideline-Supported Use</i> of Propofol in pediatric patients. | ||
|clinicalTrials=General | |clinicalTrials= | ||
=====General===== | |||
Adverse event information is derived from controlled clinical trials and worldwide marketing experience. In the description below, rates of the more common events represent US/Canadian clinical study results. Less frequent events are also derived from publications and marketing experience in over 8 million patients; there are insufficient data to support an accurate estimate of their incidence rates. These studies were conducted using a variety of premedicants, varying lengths of surgical/diagnostic procedures, and various other anesthetic/sedative agents. Most adverse events were mild and transient | * Adverse event information is derived from controlled clinical trials and worldwide marketing experience. In the description below, rates of the more common events represent US/Canadian clinical study results. Less frequent events are also derived from publications and marketing experience in over 8 million patients; there are insufficient data to support an accurate estimate of their incidence rates. These studies were conducted using a variety of premedicants, varying lengths of surgical/diagnostic procedures, and various other anesthetic/sedative agents. Most adverse events were mild and transient. | ||
=====Anesthesia and MAC Sedation in Adult===== | |||
* The following estimates of adverse events for Propofol Injectable Emulsion include data from clinical trials in general anesthesia/MAC sedation (N=2889 adult patients). The adverse events listed below as probably causally related are those events in which the actual incidence rate in patients treated with Propofol Injectable Emulsion was greater than the comparator incidence rate in these trials. Therefore, incidence rates for anesthesia and MAC sedation in adults generally represent estimates of the percentage of clinical trial patients which appeared to have probable causal relationship. | |||
* The adverse experience profile from reports of 150 patients in the MAC sedation clinical trials is similar to the profile established with Propofol Injectable Emulsion during anesthesia (see below). During MAC sedation clinical trials, significant respiratory events included [[cough]], upper airway obstruction, [[apnea]], [[hypoventilation]], and [[dyspnea]]. | |||
=====Anesthesia in Pediatric Patients===== | |||
* Generally the adverse experience profile from reports of 506 Propofol Injectable Emulsion pediatric patients from 6 days through 16 years of age in the US/Canadian anesthesia clinical trials is similar to the profile established with Propofol Injectable Emulsion during anesthesia in adults (see Pediatric percentages [Peds %] below). Although not reported as an adverse event in clinical trials, apnea is frequently observed in pediatric patients. | |||
=====ICU Sedation in Adults===== | |||
* The following estimates of adverse events include data from clinical trials in ICU sedation (N=159 adult patients). Probably related incidence rates for ICU sedation were determined by individual case report form review. Probable causality was based upon an apparent dose response relationship and/or positive responses to rechallenge. In many instances the presence of concomitant disease and concomitant therapy made the causal relationship unknown. Therefore, incidence rates for ICU sedation generally represent estimates of the percentage of clinical trial patients which appeared to have a probable causal relationship. | |||
[[File:PROPOFOLadverse1.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | |||
[[File:Propofol adverse reactions 2.png|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | [[File:Propofol adverse reactions 2.png|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | ||
|drugInteractions=The induction dose requirements of | |drugInteractions=* The induction dose requirements of Propofol Injectable Emulsion may be reduced in patients with intramuscular or intravenous premedication, particularly with narcotics (e.g., [[morphine]], [[meperidine]], and [[fentanyl]], etc.) and combinations of opioids and sedatives (e.g., [[benzodiazepines]], [[barbiturates]], [[chloral hydrate]], [[droperidol]], etc.). These agents may increase the anesthetic or sedative effects of Propofol Injectable Emulsion and may also result in more pronounced decreases in systolic, diastolic, and mean arterial pressures and cardiac output. | ||
* During maintenance of anesthesia or sedation, the rate of Propofol Injectable Emulsion administration should be adjusted according to the desired level of anesthesia or sedation and may be reduced in the presence of supplemental analgesic agents (e.g., nitrous oxide or opioids). The concurrent administration of potent inhalational agents (e.g., [[isoflurane]], [[enflurane]], and [[halothane]]) during maintenance with Propofol Injectable Emulsion has not been extensively evaluated. These inhalational agents can also be expected to increase the anesthetic or sedative and cardiorespiratory effects of Propofol Injectable Emulsion. | |||
During maintenance of anesthesia or sedation, the rate of | * Propofol Injectable Emulsion does not cause a clinically significant change in onset, intensity or duration of action of the commonly used neuromuscular blocking agents (e.g., [[succinylcholine]] and nondepolarizing muscle relaxants). | ||
* No significant adverse interactions with commonly used premedications or drugs used during anesthesia or sedation (including a range of muscle relaxants, inhalational agents, analgesic agents, and local anesthetic agents) have been observed in adults. In pediatric patients, administration of fentanyl concomitantly with Propofol Injectable Emulsion may result in serious bradycardia. | |||
No significant adverse interactions with commonly used premedications or drugs used during anesthesia or sedation (including a range of muscle relaxants, inhalational agents, analgesic agents, and local anesthetic agents) have been observed in adults. In pediatric patients, administration of fentanyl concomitantly with | |||
|FDAPregCat=B | |FDAPregCat=B | ||
|useInPregnancyFDA=Reproduction studies have been performed in rats and rabbits at intravenous doses of 15 mg/kg/day (approximately equivalent to the recommended human induction dose on a mg/m2 basis) and have revealed no evidence of impaired fertility or harm to the fetus due to propofol. Propofol, however, has been shown to cause maternal deaths in rats and rabbits and decreased pup survival during the lactating period in dams treated with 15 mg/kg/day (approximately equivalent to the recommended human induction dose on a mg/m2 basis). The pharmacological activity (anesthesia) of the drug on the mother is probably responsible for the adverse effects seen in the offspring. There are, however, no adequate and well‑controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human responses, | |useInPregnancyFDA= | ||
|useInLaborDelivery= | * Reproduction studies have been performed in rats and rabbits at intravenous doses of 15 mg/kg/day (approximately equivalent to the recommended human induction dose on a mg/m2 basis) and have revealed no evidence of impaired fertility or harm to the fetus due to propofol. Propofol, however, has been shown to cause maternal deaths in rats and rabbits and decreased pup survival during the lactating period in dams treated with 15 mg/kg/day (approximately equivalent to the recommended human induction dose on a mg/m2 basis). The pharmacological activity (anesthesia) of the drug on the mother is probably responsible for the adverse effects seen in the offspring. There are, however, no adequate and well‑controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human responses, Propofol Injectable Emulsion should be used during pregnancy only if clearly needed. | ||
|useInNursing= | |useInLaborDelivery=* Propofol Injectable Emulsion is not recommended for obstetrics, including cesarean section deliveries. Propofol Injectable Emulsion crosses the placenta, and as with other general anesthetic agents, the administration of Propofol Injectable Emulsion may be associated with neonatal depression. | ||
|useInPed=The safety and effectiveness of | |useInNursing=* Propofol Injectable Emulsion is not recommended for use in nursing mothers because Propofol Injectable Emulsion has been reported to be excreted in human milk and the effects of oral absorption of small amounts of propofol are not known. | ||
|useInPed=* The safety and effectiveness of Propofol Injectable Emulsion have been established for induction of anesthesia in pediatric patients aged 3 years and older and for the maintenance of anesthesia aged 2 months and older. | |||
* Propofol Injectable Emulsion is not recommended for the induction of anesthesia in patients younger than 3 years of age and for the maintenance of anesthesia in patients younger than 2 months of age as safety and effectiveness have not been established. | |||
* In pediatric patients, administration of fentanyl concomitantly with Propofol Injectable Emulsion may result in serious bradycardia (see Precautions, General). | |||
In pediatric patients, administration of fentanyl concomitantly with | * Propofol Injectable Emulsion is not indicated for use in pediatric patients for ICU sedation or for MAC sedation for surgical, nonsurgical or diagnostic procedures as safety and effectiveness have not been established. | ||
* There have been anecdotal reports of serious adverse events and death in pediatric patients with upper respiratory tract infections receiving Propofol Injectable Emulsion for ICU sedation. | |||
* In one multicenter clinical trial of ICU sedation in critically ill pediatric patients that excluded patients with upper respiratory tract infections, the incidence of mortality observed in patients who received Propofol Injectable Emulsion (n=222) was 9%, while that for patients who received standard sedative agents (n=105) was 4%. While causality has not been established, Propofol Injectable Emulsion is not indicated for sedation in pediatric patients until further studies have been performed to document its safety in that population (see Clinical pharmacology, Pharmacokinetics, Pediatric Patients and Dosage and administration). | |||
* In pediatric patients, abrupt discontinuation of Propofol Injectable Emulsion following prolonged infusion may result in flushing of the hands and feet, agitation, tremulousness and hyperirritability. Increased incidences of bradycardia (5%), agitation (4%), and jitteriness (9%) have also been observed. | |||
There have been anecdotal reports of serious adverse events and death in pediatric patients with upper respiratory tract infections receiving | |useInGeri=* The effect of age on induction dose requirements for propofol was assessed in an open-label study involving 211 unpremedicated patients with approximately 30 patients in each decade between the ages of 16 and 80. The average dose to induce anesthesia was calculated for patients up to 54 years of age and for patients 55 years of age or older. The average dose to induce anesthesia in patients up to 54 years of age was 1.99 mg/kg and in patients above 54 it was 1.66 mg/kg. Subsequent clinical studies have demonstrated lower dosing requirements for subjects greater than 60 years of age. | ||
* A lower induction dose and a slower maintenance rate of administration of Propofol Injectable Emulsion should be used in elderly patients. In this group of patients, rapid (single or repeated) bolus administration should not be used in order to minimize undesirable cardiorespiratory depression including [[hypotension]], [[apnea]], airway obstruction, and/or oxygen desaturation. All dosing should be titrated according to patient condition and response (see Dosage and Administration, Elderly, Debilitated or ASA-PS III or IV Patients and Clinical Pharmacology, Geriatrics). | |||
In one multicenter clinical trial of ICU sedation in critically ill pediatric patients that excluded patients with upper respiratory tract infections, the incidence of mortality observed in patients who received | |overdose=* If overdosage occurs, Propofol Injectable Emulsion administration should be discontinued immediately. Overdosage is likely to cause cardiorespiratory depression. Respiratory depression should be treated by artificial ventilation with oxygen. Cardiovascular depression may require repositioning of the patient by raising the patient's legs, increasing the flow rate of intravenous fluids, and administering pressor agents and/or [[anticholinergic]] agents. | ||
In pediatric patients, abrupt discontinuation of | |||
|useInGeri=The effect of age on induction dose requirements for propofol was assessed in an open-label study involving 211 unpremedicated patients with approximately 30 patients in each decade between the ages of 16 and 80. The average dose to induce anesthesia was calculated for patients up to 54 years of age and for patients 55 years of age or older. The average dose to induce anesthesia in patients up to 54 years of age was 1.99 mg/kg and in patients above 54 it was 1.66 mg/kg. Subsequent clinical studies have demonstrated lower dosing requirements for subjects greater than 60 years of age. | |||
A lower induction dose and a slower maintenance rate of administration of | |||
|overdose=If overdosage occurs, | |||
|drugBox={{drugbox2 | |drugBox={{drugbox2 | ||
| Watchedfields = changed | | Watchedfields = changed | ||
| Line 126: | Line 107: | ||
| routes_of_administration = [[Intravenous therapy|Intravenous]] | | routes_of_administration = [[Intravenous therapy|Intravenous]] | ||
}} | }} | ||
|structure=DIPRIVAN® (Propofol) Injectable Emulsion, USP is a sterile, nonpyrogenic emulsion containing 10 mg/mL of propofol suitable for intravenous administration. Propofol is chemically described as 2,6‑diisopropylphenol. The structural formula is: | |structure=* DIPRIVAN® (Propofol) Injectable Emulsion, USP is a sterile, nonpyrogenic emulsion containing 10 mg/mL of propofol suitable for intravenous administration. Propofol is chemically described as 2,6‑diisopropylphenol. The structural formula is: | ||
[[File:PROPOFOL structure.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | [[File:PROPOFOL structure.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | ||
Propofol is slightly soluble in water and, thus, is formulated in a white, oil-in-water emulsion. The pKa is 11. The octanol/water partition coefficient for propofol is 6761:1 at a pH of 6 to 8.5. In addition to the active component, propofol, the formulation also contains soybean oil (100 mg/mL), glycerol (22.5 mg/mL), egg lecithin (12 mg/mL); and disodium edetate (0.005%); with sodium hydroxide to adjust pH. | * Propofol is slightly soluble in water and, thus, is formulated in a white, oil-in-water emulsion. The pKa is 11. The octanol/water partition coefficient for propofol is 6761:1 at a pH of 6 to 8.5. In addition to the active component, propofol, the formulation also contains soybean oil (100 mg/mL), glycerol (22.5 mg/mL), egg lecithin (12 mg/mL); and disodium edetate (0.005%); with sodium hydroxide to adjust pH. Propofol Injectable Emulsion, USP is isotonic and has a pH of 7 to 8.5. | ||
|PD=Pharmacodynamic properties of propofol are dependent upon the therapeutic blood propofol concentrations. Steady-state propofol blood concentrations are generally proportional to infusion rates. Undesirable side effects, such as cardiorespiratory depression, are likely to occur at higher blood concentrations which result from bolus dosing or rapid increases in infusion rates. An adequate interval (3 to 5 minutes) must be allowed between dose adjustments in order to assess clinical effects | |PD=* Pharmacodynamic properties of propofol are dependent upon the therapeutic blood propofol concentrations. Steady-state propofol blood concentrations are generally proportional to infusion rates. Undesirable side effects, such as cardiorespiratory depression, are likely to occur at higher blood concentrations which result from bolus dosing or rapid increases in infusion rates. An adequate interval (3 to 5 minutes) must be allowed between dose adjustments in order to assess clinical effects. | ||
* The hemodynamic effects of Propofol Injectable Emulsion during induction of anesthesia vary. If spontaneous ventilation is maintained, the major cardiovascular effect is arterial hypotension (sometimes greater than a 30% decrease) with little or no change in heart rate and no appreciable decrease in cardiac output. If ventilation is assisted or controlled (positive pressure ventilation), there is an increase in the incidence and the degree of depression of cardiac output. Addition of an opioid, used as a premedicant, further decreases cardiac output and respiratory drive. | |||
* If anesthesia is continued by infusion of Propofol Injectable Emulsion, the stimulation of [[endotracheal intubation]] and surgery may return arterial pressure towards normal. However, cardiac output may remain depressed. Comparative clinical studies have shown that the hemodynamic effects of Propofol Injectable Emulsion during induction of anesthesia are generally more pronounced than with other intravenous (IV) induction agents. | |||
The figure below illustrates the fall of plasma propofol levels following infusions of various durations to provide ICU sedation. | * Induction of anesthesia with Propofol Injectable Emulsion is frequently associated with apnea in both adults and pediatric patients. In adult patients who received Propofol Injectable Emulsion (2 to 2.5 mg/kg), apnea lasted less than 30 seconds in 7% of patients, 30 to 60 seconds in 24% of patients, and more than 60 seconds in 12% of patients. In pediatric patients from birth through 16 years of age assessable for apnea who received bolus doses of Propofol Injectable Emulsion (1 to 3.6 mg/kg), apnea lasted less than 30 seconds in 12% of patients, 30 to 60 seconds in 10% of patients, and more than 60 seconds in 5% of patients. | ||
* During maintenance of general anesthesia, Propofol Injectable Emulsion causes a decrease in spontaneous minute ventilation usually associated with an increase in carbon dioxide tension which may be marked depending upon the rate of administration and concurrent use of other medications (e.g., [[opioids]], [[sedatives]], etc.). | |||
* During monitored anesthesia care (MAC) sedation, attention must be given to the cardiorespiratory effects of Propofol Injectable Emulsion. [[Hypotension]], [[oxyhemoglobin]] desaturation, apnea, and airway obstruction can occur, especially following a rapid bolus of Propofol Injectable Emulsion. During initiation of MAC sedation, slow infusion or slow injection techniques are preferable over rapid bolus administration. During maintenance of MAC sedation, a variable rate infusion is preferable over intermittent bolus administration in order to minimize undesirable cardiorespiratory effects. In the elderly, debilitated, or ASA-PS III or IV patients, rapid (single or repeated) bolus dose administration should not be used for MAC sedation (see Warnings). | |||
* Clinical and preclinical studies suggest that Propofol Injectable Emulsion is rarely associated with elevation of plasma histamine levels. | |||
* Preliminary findings in patients with normal intraocular pressure indicate that Propofol Injectable Emulsion produces a decrease in intraocular pressure which may be associated with a concomitant decrease in systemic vascular resistance. | |||
* Clinical studies indicate that Propofol Injectable Emulsion when used in combination with hypocarbia increases cerebrovascular resistance and decreases cerebral blood flow, cerebral metabolic oxygen consumption, and [[intracranial pressure]]. Propofol Injectable Emulsion does not affect cerebrovascular reactivity to changes in arterial carbon dioxide tension (see Clinical Trials, Neuroanesthesia). | |||
* Clinical studies indicate that Propofol Injectable Emulsion does not suppress the adrenal response to ACTH. | |||
* Animal studies and limited experience in susceptible patients have not indicated any propensity of Propofol Injectable Emulsion to induce malignant hyperthermia. | |||
* Hemosiderin deposits have been observed in the livers of dogs receiving Propofol Injectable Emulsion containing 0.005% disodium edetate over a four-week period; the clinical significance of this is unknown. | |||
|PK=* The pharmacokinetics of propofol are well described by a three compartment linear model with compartments representing the plasma, rapidly equilibrating tissues, and slowly equilibrating tissues. | |||
* Following an IV bolus dose, there is rapid equilibration between the plasma and the brain, accounting for the rapid onset of anesthesia. | |||
* Plasma levels initially decline rapidly as a result of both distribution and metabolic clearance. Distribution accounts for about half of this decline following a bolus of propofol. However, distribution is not constant over time, but decreases as body tissues equilibrate with plasma and become saturated. The rate at which equilibration occurs is a function of the rate and duration of the infusion. When equilibration occurs there is no longer a net transfer of propofol between tissues and plasma. | |||
* Discontinuation of the recommended doses of Propofol Injectable Emulsion after the maintenance of anesthesia for approximately one hour, or for sedation in the ICU for one day, results in a prompt decrease in blood propofol concentrations and rapid awakening. Longer infusions (10 days of ICU sedation) result in accumulation of significant tissue stores of propofol, such that the reduction in circulating propofol is slowed and the time to awakening is increased. | |||
* By daily titration of Propofol Injectable Emulsion dosage to achieve only the minimum effective therapeutic concentration, rapid awakening within 10 to 15 minutes can occur even after long-term administration. If, however, higher than necessary infusion levels have been maintained for a long time, propofol redistribution from fat and muscle to the plasma can be significant and slow recovery. | |||
* The figure below illustrates the fall of plasma propofol levels following infusions of various durations to provide ICU sedation. | |||
[[File:PROPOFOLpharmacokinetics 1.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | [[File:PROPOFOLpharmacokinetics 1.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | ||
The large contribution of distribution (about 50%) to the fall of propofol plasma levels following brief infusions means that after very long infusions a reduction in the infusion rate is appropriate by as much as half the initial infusion rate in order to maintain a constant plasma level. Therefore, failure to reduce the infusion rate in patients receiving | * The large contribution of distribution (about 50%) to the fall of propofol plasma levels following brief infusions means that after very long infusions a reduction in the infusion rate is appropriate by as much as half the initial infusion rate in order to maintain a constant plasma level. Therefore, failure to reduce the infusion rate in patients receiving Propofol Injectable Emulsion for extended periods may result in excessively high blood concentrations of the drug. Thus, titration to clinical response and daily evaluation of sedation levels are important during use of Propofol Injectable Emulsion infusion for ICU sedation. | ||
Propofol clearance ranges from 23 to 50 mL/kg/min (1.6 to 3.4 L/min in 70 kg adults). It is chiefly eliminated by hepatic conjugation to inactive metabolites which are excreted by the kidney. A glucuronide conjugate accounts for about 50% of the administered dose. Propofol has a steady-state volume of distribution (10-day infusion) approaching 60 L/kg in healthy adults. A difference in pharmacokinetics due to gender has not been observed. The terminal half-life of propofol after a 10-day infusion is 1 to 3 days. | =====Adults===== | ||
* Propofol clearance ranges from 23 to 50 mL/kg/min (1.6 to 3.4 L/min in 70 kg adults). It is chiefly eliminated by hepatic conjugation to inactive metabolites which are excreted by the kidney. A glucuronide conjugate accounts for about 50% of the administered dose. Propofol has a steady-state volume of distribution (10-day infusion) approaching 60 L/kg in healthy adults. A difference in pharmacokinetics due to gender has not been observed. The terminal half-life of propofol after a 10-day infusion is 1 to 3 days. | |||
Geriatrics | =====Geriatrics===== | ||
* With increasing patient age, the dose of propofol needed to achieve a defined anesthetic end point (dose-requirement) decreases. This does not appear to be an age-related change in pharmacodynamics or brain sensitivity, as measured by EEG burst suppression. With increasing patient age, pharmacokinetic changes are such that, for a given IV bolus dose, higher peak plasma concentrations occur, which can explain the decreased dose requirement. These higher peak plasma concentrations in the elderly can predispose patients to cardiorespiratory effects including hypotension, apnea, airway obstruction, and/or arterial oxygen desaturation. The higher plasma levels reflect an age-related decrease in volume of distribution and intercompartmental clearance. Lower doses are therefore recommended for initiation and maintenance of sedation and anesthesia in elderly patients (see DOSAGE AND ADMINISTRATION). | |||
=====Pediatrics===== | |||
* The pharmacokinetics of propofol were studied in children between 3 and 12 years of age who received Propofol Injectable Emulsion for periods of approximately 1 to 2 hours. The observed distribution and clearance of propofol in these children were similar to adults. | |||
=====Organ Failure===== | |||
* The pharmacokinetics of propofol do not appear to be different in people with chronic hepatic cirrhosis or chronic renal impairment compared to adults with normal hepatic and renal function. The effects of acute hepatic or renal failure on the pharmacokinetics of propofol have not been studied. | |||
|howSupplied=Propofol Injectable Emulsion, USP is available as follows: | |||
Organ Failure | |||
The pharmacokinetics of propofol do not appear to be different in people with chronic hepatic cirrhosis or chronic renal impairment compared to adults with normal hepatic and renal function. The effects of acute hepatic or renal failure on the pharmacokinetics of propofol have not been studied. | |||
|howSupplied= | |||
[[File:PROPOFOL how supplied.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | [[File:PROPOFOL how supplied.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | ||
Propofol undergoes oxidative degradation, in the presence of oxygen, and is therefore packaged under nitrogen to eliminate this degradation path. | Propofol undergoes oxidative degradation, in the presence of oxygen, and is therefore packaged under nitrogen to eliminate this degradation path. | ||
|storage=Store between 4° to 25°C (40° to 77°F). Do not freeze. Shake well before use. | |storage=* Store between 4° to 25°C (40° to 77°F). Do not freeze. Shake well before use. | ||
: All trademarks are the property of Fresenius Kabi USA, LLC. | |||
All trademarks are the property of Fresenius Kabi USA, LLC. | : PREMIERPro™Rx is a trademark of Premier, Inc., used under license. | ||
: Manufactured for: | |||
: Fresenius Kabi USA, LLC | |||
: Lake Zurich, IL 60047 | |||
PREMIERPro™Rx is a trademark of Premier, Inc., used under license. | : January 2014 | ||
: logo | |||
Manufactured for: | |||
Fresenius Kabi USA, LLC | |||
Lake Zurich, IL 60047 | |||
January 2014 | |||
logo | |||
|alcohol=Alcohol-Propofol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Propofol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | |||
{{LabelImage | |||
|fileName=Propofol Label.png | |||
}} | }} | ||
Latest revision as of 22:39, 24 June 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
For Intravenous Administration
See full prescribing information for complete Boxed Warning.
Rx only
|
Overview
Propofol is a general anesthetic that is FDA approved for the {{{indicationType}}} of general anesthesia, monitored anesthesia care sedation, sedation for a mechanically ventilated patient, intensive care unit. There is a Black Box Warning for this drug as shown here. Common adverse reactions include dermatologic: injection site pain (up to 28.5% ), gastrointestinal: nausea and vomiting (2% to 2.5% ), musculoskeletal: involuntary movement, muscle (17%).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- General anesthesia: (healthy adults less than 55 years of age) induction, 40 mg IV every 10 seconds until induction onset (2 to 2.5 mg/kg); dose varies for age and surgery type.
- General anesthesia: (healthy adults less than 55 years of age) maintenance, 100 to 200 mcg/kg/min IV infusion (6 to 12 mg/kg/hr); dose varies for age and surgery type.
- General anesthesia: (healthy adults less than 55 years of age) maintenance, 20 to 50 mg increments IV bolus as needed.
- Migraine: 10 mg slow IV push, every 5 to 10 minutes, max 80 mg; mixed with 1 mL lidocaine 2% per 10 mL of propofol 1%; infuse at rate of 1 mL/10 sec.
- Monitored anesthesia care sedation: (healthy adults less than 55 years of age) MAC initiation: 100 to 150 mcg/kg/min (6 to 9 mg/kg/hr) IV infusion or 0.5 mg/kg slow IV injection for 3 to 5 min followed immediately by maintenance infusion.
- Monitored anesthesia care sedation: (healthy adults less than 55 years of age) MAC maintenance: 25 to 75 mcg/kg/min (1.5 to 4.5 mg/kg/hr) IV infusion or 10 to 20 mg incremental IV bolus doses.
- Procedural sedation: 1 mg/kg IV followed by 0.5 mg/kg every 3 to 5 minutes as needed for sedation.
- Sedation for a mechanically ventilated patient, Intensive care unit: 5 mcg/kg/min (0.3 mg/kg/hr) IV infusion for 5 min then titrate in 5 to 10 mcg/kg/min (0.3 to 0.6 mg/kg/hr) increments to achieve desired level of sedation; allow minimum of 5 min between dose adjustments; usual maintenance rates 5 to 50 mcg/kg/min (0.3 to 3 mg/kg/hr) or higher.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Propofol in adult patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Propofol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Propofol FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Propofol in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Propofol in pediatric patients.
Contraindications
There is limited information regarding Propofol Contraindications in the drug label.
Warnings
|
For Intravenous Administration
See full prescribing information for complete Boxed Warning.
Rx only
|
There is limited information regarding Propofol Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
General
- Adverse event information is derived from controlled clinical trials and worldwide marketing experience. In the description below, rates of the more common events represent US/Canadian clinical study results. Less frequent events are also derived from publications and marketing experience in over 8 million patients; there are insufficient data to support an accurate estimate of their incidence rates. These studies were conducted using a variety of premedicants, varying lengths of surgical/diagnostic procedures, and various other anesthetic/sedative agents. Most adverse events were mild and transient.
Anesthesia and MAC Sedation in Adult
- The following estimates of adverse events for Propofol Injectable Emulsion include data from clinical trials in general anesthesia/MAC sedation (N=2889 adult patients). The adverse events listed below as probably causally related are those events in which the actual incidence rate in patients treated with Propofol Injectable Emulsion was greater than the comparator incidence rate in these trials. Therefore, incidence rates for anesthesia and MAC sedation in adults generally represent estimates of the percentage of clinical trial patients which appeared to have probable causal relationship.
- The adverse experience profile from reports of 150 patients in the MAC sedation clinical trials is similar to the profile established with Propofol Injectable Emulsion during anesthesia (see below). During MAC sedation clinical trials, significant respiratory events included cough, upper airway obstruction, apnea, hypoventilation, and dyspnea.
Anesthesia in Pediatric Patients
- Generally the adverse experience profile from reports of 506 Propofol Injectable Emulsion pediatric patients from 6 days through 16 years of age in the US/Canadian anesthesia clinical trials is similar to the profile established with Propofol Injectable Emulsion during anesthesia in adults (see Pediatric percentages [Peds %] below). Although not reported as an adverse event in clinical trials, apnea is frequently observed in pediatric patients.
ICU Sedation in Adults
- The following estimates of adverse events include data from clinical trials in ICU sedation (N=159 adult patients). Probably related incidence rates for ICU sedation were determined by individual case report form review. Probable causality was based upon an apparent dose response relationship and/or positive responses to rechallenge. In many instances the presence of concomitant disease and concomitant therapy made the causal relationship unknown. Therefore, incidence rates for ICU sedation generally represent estimates of the percentage of clinical trial patients which appeared to have a probable causal relationship.
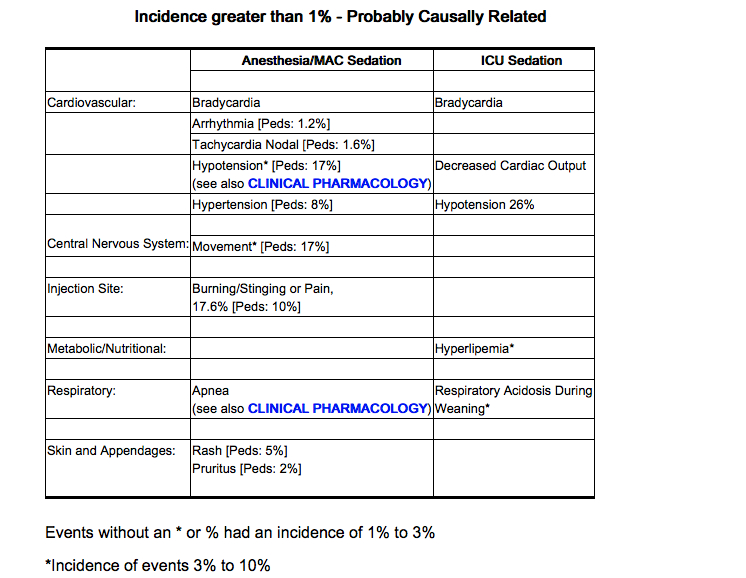
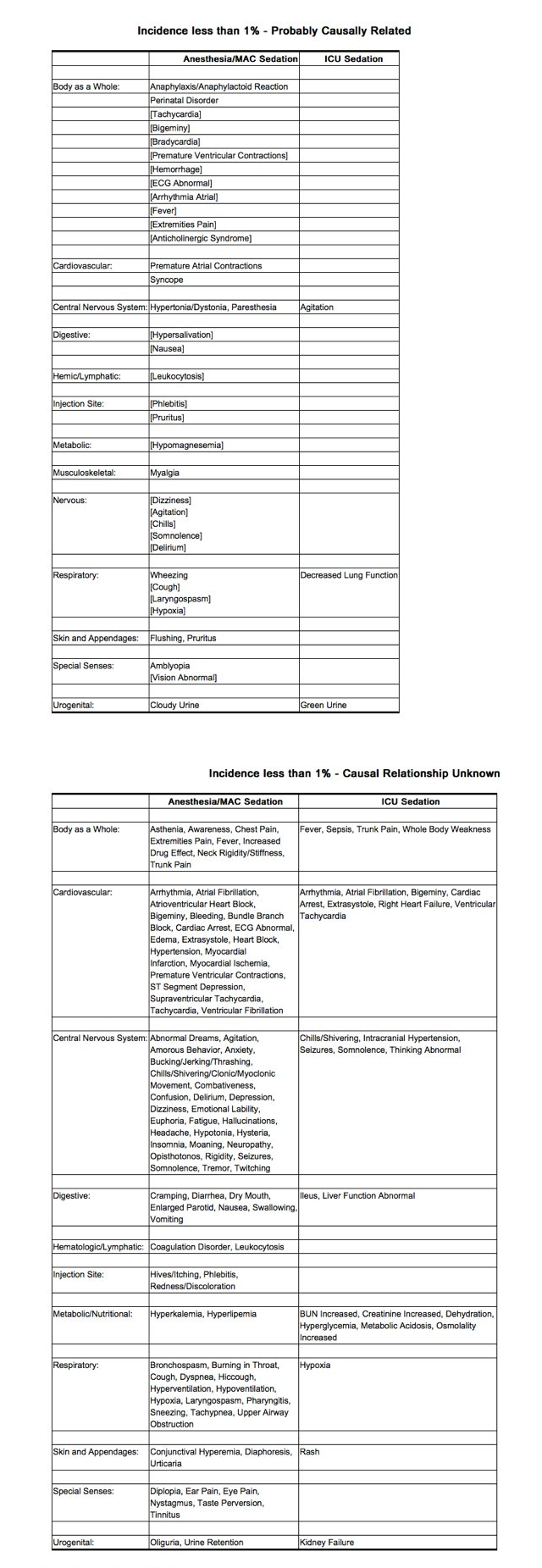
Postmarketing Experience
There is limited information regarding Propofol Postmarketing Experience in the drug label.
Drug Interactions
- The induction dose requirements of Propofol Injectable Emulsion may be reduced in patients with intramuscular or intravenous premedication, particularly with narcotics (e.g., morphine, meperidine, and fentanyl, etc.) and combinations of opioids and sedatives (e.g., benzodiazepines, barbiturates, chloral hydrate, droperidol, etc.). These agents may increase the anesthetic or sedative effects of Propofol Injectable Emulsion and may also result in more pronounced decreases in systolic, diastolic, and mean arterial pressures and cardiac output.
- During maintenance of anesthesia or sedation, the rate of Propofol Injectable Emulsion administration should be adjusted according to the desired level of anesthesia or sedation and may be reduced in the presence of supplemental analgesic agents (e.g., nitrous oxide or opioids). The concurrent administration of potent inhalational agents (e.g., isoflurane, enflurane, and halothane) during maintenance with Propofol Injectable Emulsion has not been extensively evaluated. These inhalational agents can also be expected to increase the anesthetic or sedative and cardiorespiratory effects of Propofol Injectable Emulsion.
- Propofol Injectable Emulsion does not cause a clinically significant change in onset, intensity or duration of action of the commonly used neuromuscular blocking agents (e.g., succinylcholine and nondepolarizing muscle relaxants).
- No significant adverse interactions with commonly used premedications or drugs used during anesthesia or sedation (including a range of muscle relaxants, inhalational agents, analgesic agents, and local anesthetic agents) have been observed in adults. In pediatric patients, administration of fentanyl concomitantly with Propofol Injectable Emulsion may result in serious bradycardia.
Use in Specific Populations
Pregnancy
- Reproduction studies have been performed in rats and rabbits at intravenous doses of 15 mg/kg/day (approximately equivalent to the recommended human induction dose on a mg/m2 basis) and have revealed no evidence of impaired fertility or harm to the fetus due to propofol. Propofol, however, has been shown to cause maternal deaths in rats and rabbits and decreased pup survival during the lactating period in dams treated with 15 mg/kg/day (approximately equivalent to the recommended human induction dose on a mg/m2 basis). The pharmacological activity (anesthesia) of the drug on the mother is probably responsible for the adverse effects seen in the offspring. There are, however, no adequate and well‑controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human responses, Propofol Injectable Emulsion should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Propofol in women who are pregnant.
Labor and Delivery
- Propofol Injectable Emulsion is not recommended for obstetrics, including cesarean section deliveries. Propofol Injectable Emulsion crosses the placenta, and as with other general anesthetic agents, the administration of Propofol Injectable Emulsion may be associated with neonatal depression.
Nursing Mothers
- Propofol Injectable Emulsion is not recommended for use in nursing mothers because Propofol Injectable Emulsion has been reported to be excreted in human milk and the effects of oral absorption of small amounts of propofol are not known.
Pediatric Use
- The safety and effectiveness of Propofol Injectable Emulsion have been established for induction of anesthesia in pediatric patients aged 3 years and older and for the maintenance of anesthesia aged 2 months and older.
- Propofol Injectable Emulsion is not recommended for the induction of anesthesia in patients younger than 3 years of age and for the maintenance of anesthesia in patients younger than 2 months of age as safety and effectiveness have not been established.
- In pediatric patients, administration of fentanyl concomitantly with Propofol Injectable Emulsion may result in serious bradycardia (see Precautions, General).
- Propofol Injectable Emulsion is not indicated for use in pediatric patients for ICU sedation or for MAC sedation for surgical, nonsurgical or diagnostic procedures as safety and effectiveness have not been established.
- There have been anecdotal reports of serious adverse events and death in pediatric patients with upper respiratory tract infections receiving Propofol Injectable Emulsion for ICU sedation.
- In one multicenter clinical trial of ICU sedation in critically ill pediatric patients that excluded patients with upper respiratory tract infections, the incidence of mortality observed in patients who received Propofol Injectable Emulsion (n=222) was 9%, while that for patients who received standard sedative agents (n=105) was 4%. While causality has not been established, Propofol Injectable Emulsion is not indicated for sedation in pediatric patients until further studies have been performed to document its safety in that population (see Clinical pharmacology, Pharmacokinetics, Pediatric Patients and Dosage and administration).
- In pediatric patients, abrupt discontinuation of Propofol Injectable Emulsion following prolonged infusion may result in flushing of the hands and feet, agitation, tremulousness and hyperirritability. Increased incidences of bradycardia (5%), agitation (4%), and jitteriness (9%) have also been observed.
Geriatic Use
- The effect of age on induction dose requirements for propofol was assessed in an open-label study involving 211 unpremedicated patients with approximately 30 patients in each decade between the ages of 16 and 80. The average dose to induce anesthesia was calculated for patients up to 54 years of age and for patients 55 years of age or older. The average dose to induce anesthesia in patients up to 54 years of age was 1.99 mg/kg and in patients above 54 it was 1.66 mg/kg. Subsequent clinical studies have demonstrated lower dosing requirements for subjects greater than 60 years of age.
- A lower induction dose and a slower maintenance rate of administration of Propofol Injectable Emulsion should be used in elderly patients. In this group of patients, rapid (single or repeated) bolus administration should not be used in order to minimize undesirable cardiorespiratory depression including hypotension, apnea, airway obstruction, and/or oxygen desaturation. All dosing should be titrated according to patient condition and response (see Dosage and Administration, Elderly, Debilitated or ASA-PS III or IV Patients and Clinical Pharmacology, Geriatrics).
Gender
There is no FDA guidance on the use of Propofol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Propofol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Propofol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Propofol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Propofol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Propofol in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Propofol Administration in the drug label.
Monitoring
There is limited information regarding Propofol Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Propofol and IV administrations.
Overdosage
- If overdosage occurs, Propofol Injectable Emulsion administration should be discontinued immediately. Overdosage is likely to cause cardiorespiratory depression. Respiratory depression should be treated by artificial ventilation with oxygen. Cardiovascular depression may require repositioning of the patient by raising the patient's legs, increasing the flow rate of intravenous fluids, and administering pressor agents and/or anticholinergic agents.
Pharmacology
| |
| |
Propofol
| |
| Systematic (IUPAC) name | |
| 2,6-diisopropylphenol | |
| Identifiers | |
| CAS number | |
| ATC code | N01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 178.271 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | NA |
| Protein binding | 95 to 99% |
| Metabolism | Hepatic glucuronidation |
| Half life | 30 to 60 min |
| Excretion | Hepatic |
| Therapeutic considerations | |
| Pregnancy cat. |
B (U.S.), C (Au) |
| Legal status |
Prescription Only (S4)(AU) ℞-only (U.S.) |
| Routes | Intravenous |
Mechanism of Action
There is limited information regarding Propofol Mechanism of Action in the drug label.
Structure
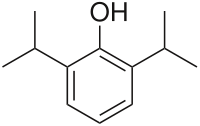
- DIPRIVAN® (Propofol) Injectable Emulsion, USP is a sterile, nonpyrogenic emulsion containing 10 mg/mL of propofol suitable for intravenous administration. Propofol is chemically described as 2,6‑diisopropylphenol. The structural formula is:
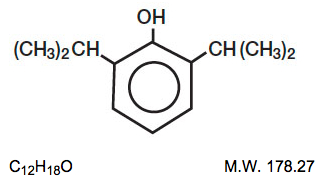
- Propofol is slightly soluble in water and, thus, is formulated in a white, oil-in-water emulsion. The pKa is 11. The octanol/water partition coefficient for propofol is 6761:1 at a pH of 6 to 8.5. In addition to the active component, propofol, the formulation also contains soybean oil (100 mg/mL), glycerol (22.5 mg/mL), egg lecithin (12 mg/mL); and disodium edetate (0.005%); with sodium hydroxide to adjust pH. Propofol Injectable Emulsion, USP is isotonic and has a pH of 7 to 8.5.
Pharmacodynamics
- Pharmacodynamic properties of propofol are dependent upon the therapeutic blood propofol concentrations. Steady-state propofol blood concentrations are generally proportional to infusion rates. Undesirable side effects, such as cardiorespiratory depression, are likely to occur at higher blood concentrations which result from bolus dosing or rapid increases in infusion rates. An adequate interval (3 to 5 minutes) must be allowed between dose adjustments in order to assess clinical effects.
- The hemodynamic effects of Propofol Injectable Emulsion during induction of anesthesia vary. If spontaneous ventilation is maintained, the major cardiovascular effect is arterial hypotension (sometimes greater than a 30% decrease) with little or no change in heart rate and no appreciable decrease in cardiac output. If ventilation is assisted or controlled (positive pressure ventilation), there is an increase in the incidence and the degree of depression of cardiac output. Addition of an opioid, used as a premedicant, further decreases cardiac output and respiratory drive.
- If anesthesia is continued by infusion of Propofol Injectable Emulsion, the stimulation of endotracheal intubation and surgery may return arterial pressure towards normal. However, cardiac output may remain depressed. Comparative clinical studies have shown that the hemodynamic effects of Propofol Injectable Emulsion during induction of anesthesia are generally more pronounced than with other intravenous (IV) induction agents.
- Induction of anesthesia with Propofol Injectable Emulsion is frequently associated with apnea in both adults and pediatric patients. In adult patients who received Propofol Injectable Emulsion (2 to 2.5 mg/kg), apnea lasted less than 30 seconds in 7% of patients, 30 to 60 seconds in 24% of patients, and more than 60 seconds in 12% of patients. In pediatric patients from birth through 16 years of age assessable for apnea who received bolus doses of Propofol Injectable Emulsion (1 to 3.6 mg/kg), apnea lasted less than 30 seconds in 12% of patients, 30 to 60 seconds in 10% of patients, and more than 60 seconds in 5% of patients.
- During maintenance of general anesthesia, Propofol Injectable Emulsion causes a decrease in spontaneous minute ventilation usually associated with an increase in carbon dioxide tension which may be marked depending upon the rate of administration and concurrent use of other medications (e.g., opioids, sedatives, etc.).
- During monitored anesthesia care (MAC) sedation, attention must be given to the cardiorespiratory effects of Propofol Injectable Emulsion. Hypotension, oxyhemoglobin desaturation, apnea, and airway obstruction can occur, especially following a rapid bolus of Propofol Injectable Emulsion. During initiation of MAC sedation, slow infusion or slow injection techniques are preferable over rapid bolus administration. During maintenance of MAC sedation, a variable rate infusion is preferable over intermittent bolus administration in order to minimize undesirable cardiorespiratory effects. In the elderly, debilitated, or ASA-PS III or IV patients, rapid (single or repeated) bolus dose administration should not be used for MAC sedation (see Warnings).
- Clinical and preclinical studies suggest that Propofol Injectable Emulsion is rarely associated with elevation of plasma histamine levels.
- Preliminary findings in patients with normal intraocular pressure indicate that Propofol Injectable Emulsion produces a decrease in intraocular pressure which may be associated with a concomitant decrease in systemic vascular resistance.
- Clinical studies indicate that Propofol Injectable Emulsion when used in combination with hypocarbia increases cerebrovascular resistance and decreases cerebral blood flow, cerebral metabolic oxygen consumption, and intracranial pressure. Propofol Injectable Emulsion does not affect cerebrovascular reactivity to changes in arterial carbon dioxide tension (see Clinical Trials, Neuroanesthesia).
- Clinical studies indicate that Propofol Injectable Emulsion does not suppress the adrenal response to ACTH.
- Animal studies and limited experience in susceptible patients have not indicated any propensity of Propofol Injectable Emulsion to induce malignant hyperthermia.
- Hemosiderin deposits have been observed in the livers of dogs receiving Propofol Injectable Emulsion containing 0.005% disodium edetate over a four-week period; the clinical significance of this is unknown.
Pharmacokinetics
- The pharmacokinetics of propofol are well described by a three compartment linear model with compartments representing the plasma, rapidly equilibrating tissues, and slowly equilibrating tissues.
- Following an IV bolus dose, there is rapid equilibration between the plasma and the brain, accounting for the rapid onset of anesthesia.
- Plasma levels initially decline rapidly as a result of both distribution and metabolic clearance. Distribution accounts for about half of this decline following a bolus of propofol. However, distribution is not constant over time, but decreases as body tissues equilibrate with plasma and become saturated. The rate at which equilibration occurs is a function of the rate and duration of the infusion. When equilibration occurs there is no longer a net transfer of propofol between tissues and plasma.
- Discontinuation of the recommended doses of Propofol Injectable Emulsion after the maintenance of anesthesia for approximately one hour, or for sedation in the ICU for one day, results in a prompt decrease in blood propofol concentrations and rapid awakening. Longer infusions (10 days of ICU sedation) result in accumulation of significant tissue stores of propofol, such that the reduction in circulating propofol is slowed and the time to awakening is increased.
- By daily titration of Propofol Injectable Emulsion dosage to achieve only the minimum effective therapeutic concentration, rapid awakening within 10 to 15 minutes can occur even after long-term administration. If, however, higher than necessary infusion levels have been maintained for a long time, propofol redistribution from fat and muscle to the plasma can be significant and slow recovery.
- The figure below illustrates the fall of plasma propofol levels following infusions of various durations to provide ICU sedation.
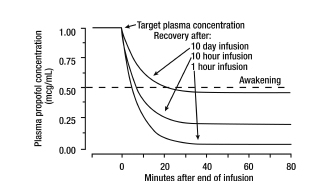
- The large contribution of distribution (about 50%) to the fall of propofol plasma levels following brief infusions means that after very long infusions a reduction in the infusion rate is appropriate by as much as half the initial infusion rate in order to maintain a constant plasma level. Therefore, failure to reduce the infusion rate in patients receiving Propofol Injectable Emulsion for extended periods may result in excessively high blood concentrations of the drug. Thus, titration to clinical response and daily evaluation of sedation levels are important during use of Propofol Injectable Emulsion infusion for ICU sedation.
Adults
- Propofol clearance ranges from 23 to 50 mL/kg/min (1.6 to 3.4 L/min in 70 kg adults). It is chiefly eliminated by hepatic conjugation to inactive metabolites which are excreted by the kidney. A glucuronide conjugate accounts for about 50% of the administered dose. Propofol has a steady-state volume of distribution (10-day infusion) approaching 60 L/kg in healthy adults. A difference in pharmacokinetics due to gender has not been observed. The terminal half-life of propofol after a 10-day infusion is 1 to 3 days.
Geriatrics
- With increasing patient age, the dose of propofol needed to achieve a defined anesthetic end point (dose-requirement) decreases. This does not appear to be an age-related change in pharmacodynamics or brain sensitivity, as measured by EEG burst suppression. With increasing patient age, pharmacokinetic changes are such that, for a given IV bolus dose, higher peak plasma concentrations occur, which can explain the decreased dose requirement. These higher peak plasma concentrations in the elderly can predispose patients to cardiorespiratory effects including hypotension, apnea, airway obstruction, and/or arterial oxygen desaturation. The higher plasma levels reflect an age-related decrease in volume of distribution and intercompartmental clearance. Lower doses are therefore recommended for initiation and maintenance of sedation and anesthesia in elderly patients (see DOSAGE AND ADMINISTRATION).
Pediatrics
- The pharmacokinetics of propofol were studied in children between 3 and 12 years of age who received Propofol Injectable Emulsion for periods of approximately 1 to 2 hours. The observed distribution and clearance of propofol in these children were similar to adults.
Organ Failure
- The pharmacokinetics of propofol do not appear to be different in people with chronic hepatic cirrhosis or chronic renal impairment compared to adults with normal hepatic and renal function. The effects of acute hepatic or renal failure on the pharmacokinetics of propofol have not been studied.
Nonclinical Toxicology
There is limited information regarding Propofol Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Propofol Clinical Studies in the drug label.
How Supplied
Propofol Injectable Emulsion, USP is available as follows:
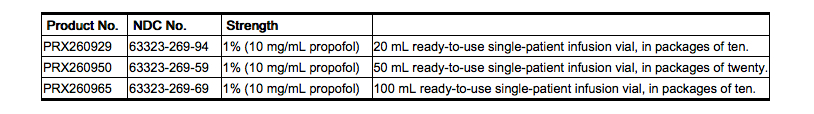
Propofol undergoes oxidative degradation, in the presence of oxygen, and is therefore packaged under nitrogen to eliminate this degradation path.
Storage
- Store between 4° to 25°C (40° to 77°F). Do not freeze. Shake well before use.
- All trademarks are the property of Fresenius Kabi USA, LLC.
- PREMIERPro™Rx is a trademark of Premier, Inc., used under license.
- Manufactured for:
- Fresenius Kabi USA, LLC
- Lake Zurich, IL 60047
- January 2014
- logo
Images
Drug Images
{{#ask: Page Name::Propofol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Propofol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Propofol Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Propofol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Propofol Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Propofol Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Propofol |Label Name=Propofol Label.png
}}