Pioglitazone/Glimepiride
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: CONGESTIVE HEART FAILURE
See full prescribing information for complete Boxed Warning.
Thiazolidinediones, including pioglitazone, which is a component of DUETACT, cause or exacerbate congestive heart failure in some patients. (5.1)
|
Overview
Pioglitazone/Glimepiride is a Thiazolidinedione that is FDA approved for the treatment of glycemic control in adults with type 2 diabetes mellitus who are already treated with a thiazolidinedione and sulfonylurea or who have inadequate glycemic control on a thiazolidinedione alone or a sulfonylurea alone. There is a Black Box Warning for this drug as shown here. Common adverse reactions include Edema, Edema of lower extremity, Hypoglycemia, Weight increased, Diarrhea, Nausea, Backache, Myalgia, Pain in limb, Headache, Urinary tract infectious disease, Pharyngitis, Sinusitis, Upper respiratory infection, Accidental injury, Influenza.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
[[Type 2 Diabetes �Mellitus]]
- Recommendations for All Patients
- DUETACT should be taken once daily with the first main meal.
- DUETACT tablets are available as a 30 mg pioglitazone plus 2 mg glimepiride or a 30 mg pioglitazone plus 4 mg glimepiride tablet. If therapy with a combination tablet containing pioglitazone and glimepiride is considered appropriate the recommended starting dose is:
- 30 mg/2 mg or 30 mg/4 mg once daily and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability,
- for patients inadequately controlled on glimepiride monotherapy: 30 mg/2 mg or 30 mg/4 mg once daily and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability,
- for patients inadequately controlled on pioglitazone monotherapy: 30 mg/2 mg once daily and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability,
- for patients who are changing from combination therapy of pioglitazone plus glimepiride as separate tablets: DUETACT should be taken at doses that are as close as possible to the dose of pioglitazone and glimepiride already being taken,
- for patients currently on a different sulfonylurea monotherapy or switching from combination therapy of pioglitazone plus a different sulfonylurea (e.g., glyburide, glipizide, chlorpropamide, tolbutamide, acetohexamide): 30 mg/2 mg once daily and adjusted after assessing adequacy of therapeutic response. Observe for hypoglycemia for one to two weeks due to the potential overlapping drug effect.
- for patients with systolic dysfunction, the lowest approved dose of DUETACT should be prescribed only after titration from 15 mg to 30 mg of pioglitazone has been safely tolerated.
- After initiation of DUETACT or with dose increase, monitor patients carefully for hypoglycemia and adverse reactions related to fluid retention such as weight gain, edema, and signs and symptoms of congestive heart failure .
- Liver tests (serum alanine and aspartate aminotransferases, alkaline phosphatase, and total bilirubin) should be obtained prior to initiating DUETACT. Routine periodic monitoring of liver tests during treatment with DUETACT is not recommended in patients without liver disease. Patients who have liver test abnormalities prior to initiation of DUETACT or who are found to have abnormal liver tests while taking DUETACT should be managed as described under Warnings and Precautions .
Concomitant Use with an Insulin Secretagogue or Insulin
- If hypoglycemia occurs in a patient coadministered DUETACT and an insulin secretagogue, the dose of the insulin secretagogue should be reduced.
- If hypoglycemia occurs in a patient coadministered DUETACT and insulin, the dose of insulin should be decreased by 10% to 25%. Further adjustments to the insulin dose should be individualized based on glycemic response.
Concomitant Use with Strong CYP2C8 Inhibitors
- Coadministration of pioglitazone and gemfibrozil, a strong CYP2C8 inhibitor, increases pioglitazone exposure approximately 3-fold. Therefore, the maximum recommended dose of pioglitazone is 15 mg daily when used in combination with gemfibrozil or other strong CYP2C8 inhibitors. If gemfibrozil or other CYP2C8 inhibitors need to co-administered, patients should switch to individual components of DUETACT because the minimum dose of pioglitazone in DUETACT exceeds 15 mg .
Concomitant Use with Colesevelam
- When colesevelam is coadministered with glimepiride, maximum plasma concentration and total exposure to glimepiride is reduced. Therefore, DUETACT should be administered at least four hours prior to colesevelam .
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pioglitazone/Glimepiride in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pioglitazone/Glimepiride in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness of DUETACT in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Pioglitazone/Glimepiride in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Pioglitazone/Glimepiride in pediatric patients.
Contraindications
- Initiation in patients with established NYHA Class III or IV heart failure .
- Use in patients with known hypersensitivity to pioglitazone, glimepiride or any other component of DUETACT .
- Use in patients with known history of an allergic reaction to sulfonamide derivatives.
Warnings
|
WARNING: CONGESTIVE HEART FAILURE
See full prescribing information for complete Boxed Warning.
Thiazolidinediones, including pioglitazone, which is a component of DUETACT, cause or exacerbate congestive heart failure in some patients. (5.1)
|
Congestive Heart Failure
Pioglitazone
Pioglitazone, like other thiazolidinediones, can cause dose-related fluid retention when used alone or in combination with other antidiabetic medications and is most common when DUETACT is used in combination with insulin. Fluid retention may lead to or exacerbate congestive heart failure. Patients should be observed for signs and symptoms of congestive heart failure. If congestive heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of DUETACT must be considered [see Boxed Warning, Contraindications (4) and Adverse Reactions (6.1)].
Hypoglycemia
Glimepiride
All sulfonylureas, including glimepiride, a component of DUETACT, can cause severe hypoglycemia [see Adverse Reactions (6.1)]. The patient's ability to concentrate and react may be impaired as a result of hypoglycemia. These impairments may present a risk in situations where these abilities are especially important, such as driving or operating other machinery. Severe hypoglycemia can lead to unconsciousness or convulsions and may result in temporary or permanent impairment of brain function or death. Patients must be educated to recognize and manage hypoglycemia. Use caution when initiating and increasing DUETACT doses in patients who may be predisposed to hypoglycemia (e.g., the elderly, patients with renal impairment, patients on other antidiabetic medications). Debilitated or malnourished patients and those with adrenal, pituitary, or hepatic impairment are particularly susceptible to the hypoglycemic action of glucose-lowering medications. Hypoglycemia is also more likely to occur when caloric intake is deficient, after severe or prolonged exercise, or when alcohol is ingested. Early warning symptoms of hypoglycemia may be different or less pronounced in patients with autonomic neuropathy, the elderly, and in patients who are taking beta-adrenergic blocking medications or other sympatholytic agents. These situations may result in severe hypoglycemia before the patient is aware of the hypoglycemia.
Hypersensitivity Reactions
Glimepiride
There have been postmarketing reports of hypersensitivity reactions in patients treated with glimepiride, a component of DUETACT, including serious reactions such as anaphylaxis, angioedema, and Stevens-Johnson Syndrome. If a hypersensitivity reaction is suspected, promptly discontinue DUETACT, assess for other potential causes for the reaction, and institute alternative treatment for diabetes.
Potential Increased Risk of Cardiovascular Mortality with Sulfonylureas
Glimepiride
The administration of oral hypoglycemic drugs has been reported to be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet plus insulin. This warning is based on the study conducted by the University Group Diabetes Program (UGDP), a long-term, prospective clinical trial designed to evaluate the effectiveness of glucose-lowering drugs in preventing or delaying vascular complications in patients with non-insulin-dependent diabetes. The study involved 823 patients who were randomly assigned to one of four treatment groups. UGDP reported that patients treated for 5 to 8 years with diet plus a fixed dose of tolbutamide (1.5 grams per day) had a rate of cardiovascular mortality approximately 2.5 times that of patients treated with diet alone. A significant increase in total mortality was not observed, but the use of tolbutamide was discontinued based on the increase in cardiovascular mortality, thus limiting the opportunity for the study to show an increase in overall mortality. Despite controversy regarding the interpretation of these results, the findings of the UGDP study provide an adequate basis for this warning. The patient should be informed of the potential risks and advantages of glimepiride tablets and of alternative modes of therapy. Although only one drug in the sulfonylurea class (tolbutamide) was included in this study, it is prudent from a safety standpoint to consider that this warning may also apply to other oral hypoglycemic drugs in this class, in view of their close similarities in mode of action and chemical structure.
Hepatic Effects
Pioglitazone
There have been postmarketing reports of fatal and non-fatal hepatic failure in patients taking pioglitazone, although the reports contain insufficient information necessary to establish the probable cause. There has been no evidence of drug-induced hepatotoxicity in the pioglitazone-controlled clinical trial database to date [see Adverse Reactions (6.1)]. Patients with type 2 diabetes may have fatty liver disease or cardiac disease with episodic congestive heart failure, both of which may cause liver test abnormalities, and they may also have other forms of liver disease, many of which can be treated or managed. Therefore, obtaining a liver test panel (serum alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase, and total bilirubin) and assessing the patient is recommended before initiating DUETACT therapy. In patients with abnormal liver tests, DUETACT should be initiated with caution. Measure liver tests promptly in patients who report symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice. In this clinical context, if the patient is found to have abnormal liver tests (ALT greater than 3 times the upper limit of the reference range), DUETACT treatment should be interrupted and investigation done to establish the probable cause. DUETACT should not be restarted in these patients without another explanation for the liver test abnormalities. Patients who have serum ALT greater than three times the reference range with serum total bilirubin greater than two times the reference range without alternative etiologies are at risk for severe drug-induced liver injury and should not be restarted on DUETACT. For patients with lesser elevations of serum ALT or bilirubin and with an alternate probable cause, treatment with DUETACT can be used with caution.
Urinary Bladder Tumors
Pioglitazone
Tumors were observed in the urinary bladder of male rats in the two-year carcinogenicity study [see Nonclinical Toxicology (13.1)]. In two 3-year trials in which pioglitazone was compared to placebo or glyburide, there were 16/3656 (0.44%) reports of bladder cancer in patients taking pioglitazone compared to 5/3679 (0.14%) in patients not taking pioglitazone. After excluding patients in whom exposure to study drug was less than one year at the time of diagnosis of bladder cancer, there were six (0.16%) cases on pioglitazone and two (0.05%) cases on placebo. A five-year interim report of an ongoing 10-year observational cohort study found a non-significant increase in the risk for bladder cancer in subjects ever exposed to pioglitazone, compared to subjects never exposed to pioglitazone (HR 1.2 [95% CI 0.9 −1.5]). Compared to never exposure, a duration of pioglitazone therapy longer than 12 months was associated with an increase in risk (HR 1.4 [95% CI 0.9 −2.1]), which reached statistical significance after more than 24 months of pioglitazone use (HR 1.4 [95% CI 1.03 −2.0]). Interim results from this study suggested that taking pioglitazone longer than 12 months increased the relative risk of developing bladder cancer in any given year by 40% which equates to an absolute increase of three cases in 10,000 (from approximately seven in 10,000 [without pioglitazone] to approximately 10 in 10,000 [with pioglitazone]). There are insufficient data to determine whether pioglitazone is a tumor promoter for urinary bladder tumors. Consequently, DUETACT should not be used in patients with active bladder cancer and the benefits of glycemic control versus unknown risks for cancer recurrence with DUETACT should be considered in patients with a prior history of bladder cancer.
Edema
Pioglitazone
In controlled clinical trials, edema was reported more frequently in patients treated with pioglitazone than in placebo-treated patients and is dose-related [see Adverse Reactions (6.1)]. In postmarketing experience, reports of new onset or worsening edema have been received. DUETACT should be used with caution in patients with edema. Because thiazolidinediones, including pioglitazone, can cause fluid retention, which can exacerbate or lead to congestive heart failure, DUETACT should be used with caution in patients at risk for congestive heart failure. Patients treated with DUETACT should be monitored for signs and symptoms of congestive heart failure [see Boxed Warning, Warnings and Precautions (5.1), and Patient Counseling Information (17)].
5.8 Fractures
Pioglitazone
In PROactive (the Prospective Pioglitazone Clinical Trial in Macrovascular Events), 5238 patients with type 2 diabetes and a history of macrovascular disease were randomized to pioglitazone (N=2605), force-titrated up to 45 mg daily or placebo (N=2633) in addition to standard of care. During a mean follow-up of 34.5 months, the incidence of bone fracture in females was 5.1% (44/870) for pioglitazone versus 2.5% (23/905) for placebo. This difference was noted after the first year of treatment and persisted during the course of the study. The majority of fractures observed in female patients were nonvertebral fractures including lower limb and distal upper limb. No increase in the incidence of fracture was observed in men treated with pioglitazone (1.7%) versus placebo (2.1%). The risk of fracture should be considered in the care of patients, especially female patients, treated with DUETACT and attention should be given to assessing and maintaining bone health according to current standards of care.
Hemolytic Anemia
Glimepiride
Sulfonylureas can cause hemolytic anemia in patients with glucose 6-phosphate dehydrogenase (G6PD) deficiency. Because DUETACT contains glimepiride, which belongs to the class of sulfonylurea agents, use caution in patients with G6PD deficiency and consider the use of a nonsulfonylurea alternative. There are also postmarketing reports of hemolytic anemia in patients receiving glimepiride who did not have known G6PD deficiency [see Adverse Reactions (6.2)].
Macular Edema
Pioglitazone
Macular edema has been reported in postmarketing experience in diabetic patients who were taking pioglitazone or another thiazolidinedione. Some patients presented with blurred vision or decreased visual acuity, but others were diagnosed on routine ophthalmologic examination. Most patients had peripheral edema at the time macular edema was diagnosed. Some patients had improvement in their macular edema after discontinuation of the thiazolidinedione. Patients with diabetes should have regular eye exams by an ophthalmologist according to current standards of care. Patients with diabetes who report any visual symptoms should be promptly referred to an ophthalmologist, regardless of the patient’s underlying medications or other physical findings [see Adverse Reactions (6.1)].
Ovulation
Pioglitazone
Therapy with pioglitazone, like other thiazolidinediones, may result in ovulation in some premenopausal anovulatory women. As a result, these patients may be at an increased risk for pregnancy while taking DUETACT [see Use in Specific Populations (8.1)]. This effect has not been investigated in clinical trials, so the frequency of this occurrence is not known. Adequate contraception in all premenopausal women treated with DUETACT is recommended.
Macrovascular Outcomes
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with DUETACT or any other antidiabetic drug.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse events reported in at least 5% of patients in the controlled 16-week clinical studies between placebo plus a sulfonylurea and pioglitazone (15 mg and 30 mg combined) plus sulfonylurea treatment arms were upper respiratory tract infection (15.5% and 16.6%), accidental injury (8.6% and 3.5%), and combined edema/peripheral edema (2.1% and 7.2%), respectively. The incidence and type of adverse events reported in at least 5% of patients in any combined treatment group from the 24-week study comparing pioglitazone 30 mg plus a sulfonylurea and pioglitazone 45 mg plus a sulfonylurea are shown in Table 1; the rate of adverse events resulting in study discontinuation between the two treatment groups was 6% and 9.7%, respectively.
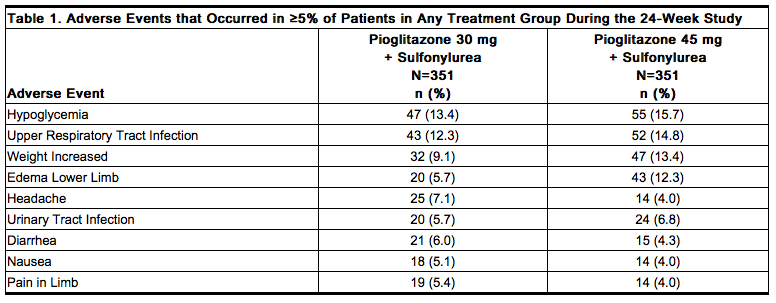
In US double-blind studies, anemia was reported in ≤2% of patients treated with pioglitazone plus a sulfonylurea .
Pioglitazone
Over 8500 patients with type 2 diabetes have been treated with pioglitazone in randomized, double-blind, controlled clinical trials, including 2605 patients with type 2 diabetes and macrovascular disease treated with pioglitazone in the PROactive clinical trial. In these trials, over 6000 patients have been treated with pioglitazone for six months or longer, over 4500 patients have been treated with pioglitazone for one year or longer, and over 3000 patients have been treated with pioglitazone for at least two years. In six pooled 16- to 26-week placebo-controlled monotherapy and 16- to 24-week add-on combination therapy trials, the incidence of withdrawals due to adverse events was 4.5% for patients treated with pioglitazone and 5.8% for comparator-treated patients. The most common adverse events leading to withdrawal were related to inadequate glycemic control, although the incidence of these events was lower (1.5%) with pioglitazone than with placebo (3.0%). In the PROactive trial, the incidence of withdrawals due to adverse events was 9.0% for patients treated with pioglitazone and 7.7% for placebo-treated patients. Congestive heart failure was the most common serious adverse event leading to withdrawal occurring in 1.3% of patients treated with pioglitazone and 0.6% of patients treated with placebo.
<��i>Common Adverse Events: 16- to 26-Week Monotherapy Trials:<��/i>
A summary of the incidence and type of common adverse events reported in three pooled 16- to 26-week placebo-controlled monotherapy trials of pioglitazone is provided in Table 2. Terms that are reported represent those that occurred at an incidence of >5% and more commonly in patients treated with pioglitazone than in patients who received placebo. None of these adverse events were related to the pioglitazone dose.

A summary of the overall incidence and types of common adverse events reported in the PROactive trial is provided in Table 3. Terms that are reported represent those that occurred at an incidence of >5% and more commonly in patients treated with pioglitazone than in patients who received placebo.

Congestive Heart Failure
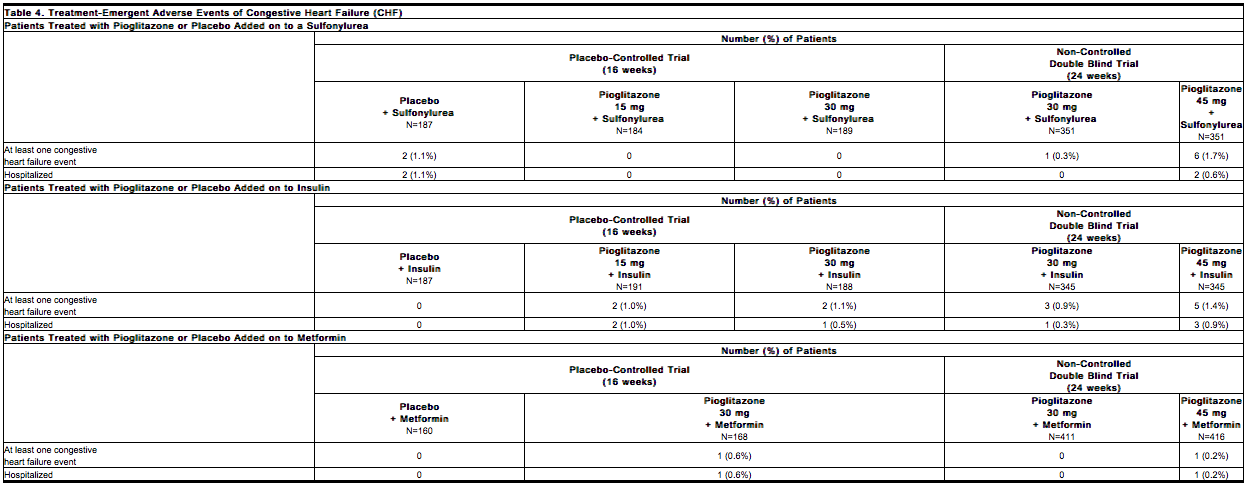
A summary of the incidence of adverse events related to congestive heart failure is provided in Table 4 for the 16- to 24-week add-on to sulfonylurea trials, for the 16- to 24-week add-on to insulin trials, and for the 16- to 24-week add-on to metformin trials. None of the events were fatal.
Patients with type 2 diabetes and NYHA class II or early class III congestive heart failure were randomized to receive 24 weeks of double-blind treatment with either pioglitazone at daily doses of 30 mg to 45 mg (n=262) or glyburide at daily doses of 10 mg to 15 mg (n=256). A summary of the incidence of adverse events related to congestive heart failure reported in this study is provided in Table 5.

Congestive heart failure events leading to hospitalization that occurred during the PROactive trial are summarized in Table 6.

<�i>Cardiovascular Safety<�/i>
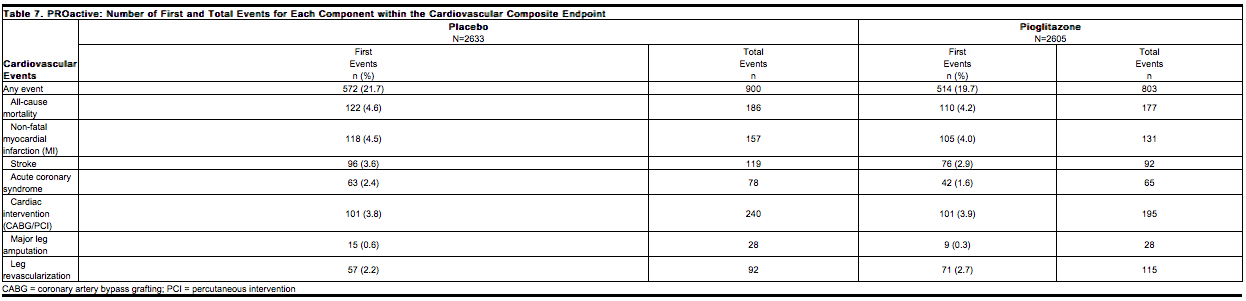
In the PROactive trial, 5238 patients with type 2 diabetes and a history of macrovascular disease were randomized to pioglitazone (N=2605), force-titrated up to 45 mg daily or placebo (N=2633) in addition to standard of care. Almost all patients (95%) were receiving cardiovascular medications (beta blockers, ACE inhibitors, angiotensin II receptor blockers, calcium channel blockers, nitrates, diuretics, aspirin, statins, and fibrates). At baseline, patients had a mean age of 62 years, mean duration of diabetes of 9.5 years, and mean HbA1c of 8.1%. Mean duration of follow-up was 34.5 months. The primary objective of this trial was to examine the effect of pioglitazone on mortality and macrovascular morbidity in patients with type 2 diabetes mellitus who were at high risk for macrovascular events. The primary efficacy variable was the time to the first occurrence of any event in a cardiovascular composite endpoint that included all-cause mortality, nonfatal myocardial infarction (MI) including silent MI, stroke, acute coronary syndrome, cardiac intervention including coronary artery bypass grafting or percutaneous intervention, major leg amputation above the ankle, and bypass surgery or revascularization in the leg. A total of 514 (19.7%) patients treated with pioglitazone and 572 (21.7%) placebo-treated patients experienced at least one event from the primary composite endpoint (hazard ratio 0.90; 95% Confidence Interval: 0.80, 1.02; p=0.10). Although there was no statistically significant difference between pioglitazone and placebo for the three-year incidence of a first event within this composite, there was no increase in mortality or in total macrovascular events with pioglitazone. The number of first occurrences and total individual events contributing to the primary composite endpoint is shown in Table 7.
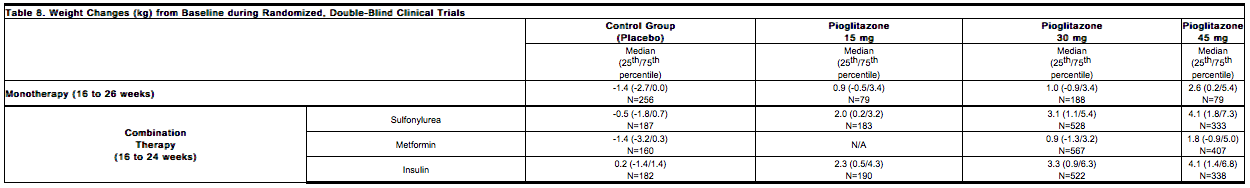
<�i>Weight Gain<�/i>
Dose-related weight gain occurs when pioglitazone is used alone or in combination with other antidiabetic medications. The mechanism of weight gain is unclear but probably involves a combination of fluid retention and fat accumulation. Tables 8 and 9 summarize the changes in body weight with pioglitazone and placebo in the 16- to 26-week randomized, double-blind monotherapy and 16- to 24-week combination add-on therapy trials and in the PROactive trial.

Edema
Edema induced from taking pioglitazone is reversible when pioglitazone is discontinued. The edema usually does not require hospitalization unless there is coexisting congestive heart failure. A summary of the frequency and types of edema adverse events occurring in clinical investigations of pioglitazone is provided in Table 10.

Hepatic Effects
There has been no evidence of pioglitazone-induced hepatotoxicity in the pioglitazone-controlled clinical trial database to date. One randomized, double-blind, 3-year trial comparing pioglitazone to glyburide as add-on to metformin and insulin therapy was specifically designed to evaluate the incidence of serum ALT elevation to greater than three times the upper limit of the reference range, measured every eight weeks for the first 48 weeks of the trial then every 12 weeks thereafter. A total of 3/1051 (0.3%) patients treated with pioglitazone and 9/1046 (0.9%) patients treated with glyburide developed ALT values greater than three times the upper limit of the reference range. None of the patients treated with pioglitazone in the pioglitazone-controlled clinical trial database to date have had a serum ALT greater than three times the upper limit of the reference range and a corresponding total bilirubin greater than two times the upper limit of the reference range, a combination predictive of the potential for severe drug-induced liver injury.
Hypoglycemia
In the pioglitazone clinical trials, adverse events of hypoglycemia were reported based on clinical judgment of the investigators and did not require confirmation with fingerstick glucose testing. In the 16-week add-on to sulfonylurea trial, the incidence of reported hypoglycemia was 3.7% with pioglitazone 30 mg and 0.5% with placebo. In the 16-week add-on to insulin trial, the incidence of reported hypoglycemia was 7.9% with pioglitazone 15 mg, 15.4% with pioglitazone 30 mg, and 4.8% with placebo. The incidence of reported hypoglycemia was higher with pioglitazone 45 mg compared to pioglitazone 30 mg in both the 24-week add-on to sulfonylurea trial (15.7% versus 13.4%) and in the 24-week add-on to insulin trial (47.8% versus 43.5%). Three patients in these four trials were hospitalized due to hypoglycemia. All three patients were receiving pioglitazone 30 mg (0.9%) in the 24-week add-on to insulin trial. An additional 14 patients reported severe hypoglycemia (defined as causing considerable interference with patient’s usual activities) that did not require hospitalization. These patients were receiving pioglitazone 45 mg in combination with sulfonylurea (N=2) or pioglitazone 30 mg or 45 mg in combination with insulin (N=12).
Urinary Bladder Tumors
Tumors were observed in the urinary bladder of male rats in the two-year carcinogenicity study [see Nonclinical Toxicology (13.1)]. In two 3-year trials in which pioglitazone was compared to placebo or glyburide, there were 16/3656 (0.44%) reports of bladder cancer in patients taking pioglitazone compared to 5/3679 (0.14%) in patients not taking pioglitazone. After excluding patients in whom exposure to study drug was less than one year at the time of diagnosis of bladder cancer, there were six (0.16%) cases on pioglitazone and two (0.05%) cases on placebo. There are too few events of bladder cancer to establish causality.
Glimepiride Adverse events that occurred in controlled clinical trials with placebo and glimepiride monotherapy, other than hypoglycemia, included: headache (7.8% and 8.2%), accidental injury (3.4% and 5.8%), flu syndrome (4.4% and 5.4%), nausea (3.4% and 5.0%) and dizziness (2.4% and 5.0%), respectively.
Hypoglycemia In a randomized, double-blind, placebo-controlled monotherapy trial of 14 weeks duration, patients already on sulfonylurea therapy underwent a 3-week washout period then were randomized to glimepiride 1 mg, 4 mg, 8 mg or placebo. Patients randomized to glimepiride 4 mg or 8 mg underwent forced-titration from an initial dose of 1 mg to these final doses, as tolerated. The overall incidence of possible hypoglycemia (defined by the presence of at least one symptom that the investigator believed might be related to hypoglycemia; a concurrent glucose measurement was not required) was 4% for glimepiride 1 mg, 17% for glimepiride 4 mg, 16% for glimepiride 8 mg and 0% for placebo. All of these events were self-treated. In a randomized, double-blind, placebo-controlled monotherapy trial of 22 weeks duration, patients received a starting dose of either 1 mg glimepiride or placebo daily. The dose of glimepiride was titrated to a target fasting plasma glucose of 90 −150 mg/dL. Final daily doses of glimepiride were 1, 2, 3, 4, 6 or 8 mg. The overall incidence of possible hypoglycemia (as defined above for the 14-week trial) for glimepiride versus placebo was 19.7% vs. 3.2%. All of these events were self-treated.
Weight Gain
Glimepiride, like all sulfonylureas, can cause weight gain.
Allergic Reactions
In clinical trials, allergic reactions, such as pruritus, erythema, urticaria, and morbilliform or maculopapular eruptions, occurred in less than 1% of glimepiride-treated patients. These may resolve despite continued treatment with glimepiride. There are postmarketing reports of more serious allergic reactions (e.g., dyspnea, hypotension, shock) [see Warnings and Precautions (5.3)].
Laboratory Tests
Elevated Serum Alanine Aminotransferase (ALT)
In 11 pooled placebo-controlled trials of glimepiride, 1.9% of glimepiride-treated patients and 0.8% of placebo-treated patients developed serum ALT greater than two times the upper limit of the reference range.
Laboratory Abnormalities
Pioglitazone
Hematologic Effects
Pioglitazone may cause decreases in hemoglobin and hematocrit. In placebo-controlled monotherapy trials, mean hemoglobin values declined by 2% to 4% in patients treated with pioglitazone compared with a mean change in hemoglobin of -1% to +1% in placebo-treated patients. These changes primarily occurred within the first 4 to 12 weeks of therapy and remained relatively constant thereafter. These changes may be related to increased plasma volume associated with pioglitazone therapy and are not likely to be associated with any clinically significant hematologic effects.
Creatine Phosphokinase
During protocol-specified measurement of serum creatine phosphokinase (CPK) in pioglitazone clinical trials, an isolated elevation in CPK to greater than 10 times the upper limit of the reference range was noted in nine (0.2%) patients treated with pioglitazone (values of 2150 to 11400 IU/L) and in no comparator-treated patients. Six of these nine patients continued to receive pioglitazone, two patients were noted to have the CPK elevation on the last day of dosing and one patient discontinued pioglitazone due to the elevation. These elevations resolved without any apparent clinical sequelae. The relationship of these events to pioglitazone therapy is unknown.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of pioglitazone and glimepiride. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Pioglitazone
- New onset or worsening diabetic macular edema with decreased visual acuity .
- Fatal and nonfatal hepatic failure.
Postmarketing reports of congestive heart failure have been reported in patients treated with pioglitazone, both with and without previously known heart disease and both with and without concomitant insulin administration. In postmarketing experience, there have been reports of unusually rapid increases in weight and increases in excess of that generally observed in clinical trials. Patients who experience such increases should be assessed for fluid accumulation and volume-related events such as excessive edema and congestive heart failure .
Glimepiride
- Serious hypersensitivity reactions, including anaphylaxis, angioedema, and Stevens-Johnson Syndrome
- Hemolytic anemia in patients with and without G6PD deficiency
- Impairment of liver function (e.g. with cholestasis and jaundice), as well as hepatitis, which may progress to liver failure.
- Porphyria cutanea tarda, photosensitivity reactions and allergic vasculitis
- Leukopenia, agranulocytosis, thrombocytopenia, aplastic anemia, and pancytopenia
- Hepatic porphyria reactions and disulfiram-like reactions
- Hyponatremia and syndrome of inappropriate antidiuretic hormone secretion (SIADH), most often in patients who are on other medications or who have medical conditions known to cause hyponatremia or increase release of antidiuretic hormone
Drug Interactions
Strong CYP2C8 Inhibitors
Pioglitazone
An inhibitor of CYP2C8 (e.g., gemfibrozil) significantly increases the exposure (area under the serum concentration-time curve or AUC) and half-life (t½) of pioglitazone. Therefore, the maximum recommended dose of pioglitazone is 15 mg daily if used in combination with gemfibrozil or other strong CYP2C8 inhibitors. Since the minimum dose of pioglitazone in DUETACT exceeds 15 mg, patients taking concomitant strong CYP2C8 inhibitors should switch to individual components of DUETACT, unless the prescribing health care provider determines that the benefit of DUETACT clearly outweighs the risk of increased pioglitazone exposure .
CYP2C8 Inducers
Pioglitazone
An inducer of CYP2C8 (e.g., rifampin) may significantly decrease the exposure (AUC) of pioglitazone. Therefore, if an inducer of CYP2C8 is started or stopped during treatment with pioglitazone, changes in diabetes treatment may be needed based on clinical response without exceeding the maximum recommended daily dose of 45 mg for pioglitazone .
Miconazole
Glimepiride
A potential interaction between oral miconazole and sulfonylureas leading to severe hypoglycemia has been reported. Whether this interaction also occurs with other dosage forms of miconazole is not known.
CYP2C9 Interactions
Glimepiride
There may be an interaction between glimepiride and inhibitors (e.g., fluconazole) and inducers (e.g., rifampin) of CYP2C9. Fluconazole may inhibit the metabolism of glimepiride, causing increased plasma concentrations of glimepiride which may lead to hypoglycemia. Rifampin may induce the metabolism of glimepiride, causing decreased plasma concentrations of glimepiride which may lead to worsening glycemic control.
Concomitant Administration of Colesevelam
Glimepiride
Colesevelam can reduce the maximum plasma concentrations and total exposure of glimepiride when the two are coadministered. However, absorption is not reduced when glimepiride is administered four hours prior to colesevelam. Therefore, DUETACT should be administered at least four hours prior to colesevelam.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Pioglitazone
There are no adequate and well-controlled studies of DUETACT in pregnant women. Animal studies show increased rates of postimplantation loss, delayed development, reduced fetal weights, and delayed parturition at doses 10 to 40 times the maximum recommended human dose. DUETACT should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Clinical Considerations
Abnormal blood glucose concentrations during pregnancy are associated with a higher incidence of congenital anomalies, as well as increased neonatal morbidity and mortality. Most experts recommend the use of insulin during pregnancy to maintain blood glucose concentrations as close to normal as possible for patients with diabetes.
Animal Data In animal reproductive studies, pregnant rats and rabbits received pioglitazone at doses up to approximately 17 (rat) and 40 (rabbit) times the maximum recommended human oral dose (MRHD) based on body surface area (mg/m2); no teratogenicity was observed. Increases in embryotoxicity (increased postimplantation losses, delayed development, reduced fetal weights, and delayed parturition) occurred in rats that received oral doses approximately 10 or more times the MRHD (mg/m2 basis). No functional or behavioral toxicity was observed in rat offspring. When pregnant rats received pioglitazone during late gestation and lactation, delayed postnatal development, attributed to decreased body weight, occurred in rat offspring at oral maternal doses approximately two or more times the MRHD (mg/m2 basis). In rabbits, embryotoxicity occurred at oral doses approximately 40 times the MRHD (mg/m2 basis).
Glimepiride
Teratogenic Effects
In animal studies there was no increase in congenital anomalies, but an increase in fetal deaths occurred in rats and rabbits at glimepiride doses 50 times (rats) and 0.1 times (rabbits) the maximum recommended human dose (based on body surface area). This fetotoxicity, observed only at doses inducing maternal hypoglycemia, is believed to be directly related to the pharmacologic (hypoglycemic) action of glimepiride and has been similarly noted with other sulfonylureas. DUETACT should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Because data suggest that abnormal blood glucose during pregnancy is associated with a higher incidence of congenital abnormalities, diabetes treatment during pregnancy should maintain blood glucose as close to normal as possible.
Nonteratogenic Effects
Prolonged severe hypoglycemia (4 to 10 days) has been reported in neonates born to mothers receiving a sulfonylurea at the time of delivery.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Pioglitazone/Glimepiride in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Pioglitazone/Glimepiride during labor and delivery.
Nursing Mothers
No studies have been conducted with the combined components of DUETACT. In studies performed with the individual components, pioglitazone was secreted in the milk of lactating rats and significant concentrations of glimepiride were observed in the serum and breast milk of the dams and serum of the pups. It is not known whether pioglitazone or glimepiride are secreted in human milk. However, other sulfonylureas are excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for DUETACT to cause serious adverse reactions in nursing infants, a decision should be made to discontinue nursing or discontinue DUETACT, taking into account the importance of DUETACT to the mother.
Pediatric Use
Safety and effectiveness of DUETACT in pediatric patients have not been established. DUETACT is not recommended for use in pediatric patients based on adverse effects observed in adults, including fluid retention and congestive heart failure, fractures, and urinary bladder tumors.
Glimepiride
The pharmacokinetics, efficacy and safety of glimepiride have been evaluated in pediatric patients with type 2 diabetes as described below. Glimepiride is not recommended in pediatric patients because of its adverse effects on body weight and hypoglycemia. The pharmacokinetics of a 1 mg single dose of glimepiride was evaluated in 30 patients with type 2 diabetes (male = 7; female = 23) between ages 10 and 17 years. The mean (±SD) AUC (0-last) (339±203 ng•hr/mL), Cmax (102±48 ng/mL) and t1/2 (3.1±1.7 hours) for glimepiride were comparable to historical data from adults (AUC (0-last) 315±96 ng•hr/mL, Cmax 103±34 ng/mL and t1/2 5.3±4.1 hours). The safety and efficacy of glimepiride in pediatric patients was evaluated in a single-blind, 24-week trial that randomized 272 patients (8 to 17 years of age) with type 2 diabetes to glimepiride (n=135) or metformin (n=137). Both treatment-naïve patients (those treated with only diet and exercise for at least two weeks prior to randomization) and previously treated patients (those previously treated or currently treated with other oral antidiabetic medications for at least three months) were eligible to participate. Patients who were receiving oral antidiabetic agents at the time of study entry discontinued these medications before randomization without a washout period. Glimepiride was initiated at 1 mg, and then titrated up to 2, 4 or 8 mg (mean last dose 4 mg) through Week 12, targeting a self monitored fasting fingerstick blood glucose <126 mg/dL. Metformin was initiated at 500 mg twice daily and titrated at Week 12 up to 1000 mg twice daily (mean last dose 1365 mg). After 24 weeks, the overall mean treatment difference in HbA1c between glimepiride and metformin was 0.2%, favoring metformin (95% confidence interval -0.3% to +0.6%). Based on these results, the trial did not meet its primary objective of showing a similar reduction in HbA1c with glimepiride compared to metformin. The profile of adverse reactions in pediatric patients treated with glimepiride was similar to that observed in adults. Hypoglycemic events documented by blood glucose values <36 mg/dL were observed in 4% of pediatric patients treated with glimepiride and in 1% of pediatric patients treated with metformin. One patient in each treatment group experienced a severe hypoglycemic episode (severity was determined by the investigator based on observed signs and symptoms).
Geriatic Use
To minimize the risk of hypoglycemia, the initial dosing, dose increments, and maintenance dosage of DUETACT should be conservative. During initiation of DUETACT therapy and any subsequent dose adjustments, geriatric patients should be observed carefully for hypoglycemia.
Pioglitazone
A total of 92 patients (15.2%) treated with pioglitazone in the three pooled 16- to 26-week double-blind, placebo-controlled, monotherapy trials were ≥65 years old and two patients (0.3%) were ≥75 years old. In the two pooled 16- to 24-week add-on to sulfonylurea trials, 201 patients (18.7%) treated with pioglitazone were ≥65 years old and 19 (1.8%) were ≥75 years old. In the two pooled 16- to 24-week add-on to metformin trials, 155 patients (15.5%) treated with pioglitazone were ≥65 years old and 19 (1.9%) were ≥75 years old. In the two pooled 16- to 24-week add-on to insulin trials, 272 patients (25.4%) treated with pioglitazone were ≥65 years old and 22 (2.1%) were ≥75 years old. In PROactive, 1068 patients (41.0%) treated with pioglitazone were ≥65 years old and 42 (1.6%) were ≥75 years old. In pharmacokinetic studies with pioglitazone, no significant differences were observed in pharmacokinetic parameters between elderly and younger patients . Although clinical experiences have not identified differences in effectiveness and safety between the elderly (≥65 years) and younger patients, these conclusions are limited by small sample sizes for patients ≥75 years old.
Glimepiride
In clinical trials of glimepiride, 1053 of 3491 patients (30%) were ≥65 years of age. No overall differences in safety or effectiveness were observed between these patients and younger patients, but greater sensitivity of some older individuals cannot be ruled out. There were no significant differences in glimepiride pharmacokinetics between patients with type 2 diabetes ≤65 years (n=49) and those >65 years (n=42). Glimepiride is substantially excreted by the kidney. Elderly patients are more likely to have renal impairment. In addition, hypoglycemia may be difficult to recognize in the elderly . Use caution when initiating DUETACT and increasing the dose of DUETACT in this patient population.
Gender
There is no FDA guidance on the use of Pioglitazone/Glimepiride with respect to specific gender populations.
Race
There is no FDA guidance on the use of Pioglitazone/Glimepiride with respect to specific racial populations.
Renal Impairment
To minimize the risk of hypoglycemia, the initial dosing, dose increments and maintenance dosage of DUETACT should be conservative. During initiation of DUETACT therapy and any subsequent dose adjustments, these patients should be observed carefully for hypoglycemia. A multiple-dose titration study was conducted in 16 patients with type 2 diabetes and renal impairment using doses ranging from 1 mg to 8 mg daily for three months. Baseline creatinine clearance ranged from 10 to 60 mL/min. The pharmacokinetics of glimepiride were evaluated in the multiple-dose titration study and the results were consistent with those observed in patients enrolled in a single-dose study. In both studies, the relative total clearance of glimepiride increased when kidney function was impaired. Both studies also demonstrated that the elimination of the two major metabolites was reduced in patients with renal impairment
Hepatic Impairment
There is no FDA guidance on the use of Pioglitazone/Glimepiride in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Pioglitazone/Glimepiride in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Pioglitazone/Glimepiride in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
FDA Package Insert for Pioglitazone/Glimepiride contains no information regarding Drug Monitoring.
IV Compatibility
There is limited information about the IV Compabitility.
Overdosage
Pioglitazone
During controlled clinical trials, one case of overdose with pioglitazone was reported. A male patient took 120 mg per day for four days, then 180 mg per day for seven days. The patient denied any clinical symptoms during this period. In the event of overdosage, appropriate supportive treatment should be initiated according to the patient’s clinical signs and symptoms.
Glimepiride
An overdosage of glimepiride, as with other sulfonylureas, can produce severe hypoglycemia. Mild episodes of hypoglycemia can be treated with oral glucose. Severe hypoglycemic reactions constitute medical emergencies requiring immediate treatment. Severe hypoglycemia with coma, seizure, or neurological impairment can be treated with glucagon or intravenous glucose. Continued observation and additional carbohydrate intake may be necessary because hypoglycemia may recur after apparent clinical recovery.
Pharmacology
There is limited information regarding Pioglitazone/Glimepiride Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Pioglitazone/Glimepiride Mechanism of Action in the drug label.
Structure
There is limited information regarding Pioglitazone/Glimepiride Structure in the drug label.
Pharmacodynamics
There is limited information regarding Pioglitazone/Glimepiride Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Pioglitazone/Glimepiride Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Pioglitazone/Glimepiride Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Pioglitazone/Glimepiride Clinical Studies in the drug label.
How Supplied
There is limited information regarding Pioglitazone/Glimepiride How Supplied in the drug label.
Storage
There is limited information regarding Pioglitazone/Glimepiride Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Pioglitazone/Glimepiride |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Pioglitazone/Glimepiride |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Pioglitazone/Glimepiride Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Pioglitazone/Glimepiride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Pioglitazone/Glimepiride Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Pioglitazone/Glimepiride Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.