Penbutolol: Difference between revisions
No edit summary |
No edit summary |
||
| Line 58: | Line 58: | ||
=====Thyrotoxicosis===== | =====Thyrotoxicosis===== | ||
Beta-adrenergic blockade may mask certain clinical signs (eg, [[tachycardia]]) of [[hyperthyroidism]]. Patients suspected of developing thyrotoxicosis should be managed carefully to avoid abrupt withdrawal of ß-adrenergic receptor blockers that might precipitate a thyroid storm. | Beta-adrenergic blockade may mask certain clinical signs (eg, [[tachycardia]]) of [[hyperthyroidism]]. Patients suspected of developing thyrotoxicosis should be managed carefully to avoid abrupt withdrawal of ß-adrenergic receptor blockers that might precipitate a thyroid storm. | ||
|clinicalTrials= | |clinicalTrials=Penbutolol is usually well tolerated in properly selected patients. Most adverse effects observed during clinical trials have been mild and reversible. | ||
Table 1 lists the adverse reactions reported from 4 controlled studies conducted in the United States involving once-a-day administration of penbutolol (at doses ranging from 10 to 120 mg) as monotherapy or in combination with [[hydrochlorothiazide]]. Penbutolol doses above 40 mg/day are not, however, recommended. The table includes only those events where the prevalence rate in the penbutolol group was at least 1.5%, or where the reaction is of particular interest. | |||
Over a dose range from 10 to 40 mg, once a day, [[fatigue]], [[nausea]], and [[sexual impotence]] occurred at a greater frequency as the dose was increased. | |||
: | [[File:Penbutolol01.jpg|800px|thumbnail|left|This image is provided by the National Library of Medicine.]] | ||
{{clr}} | |||
In a double-blind clinical trial comparing In a double-blind clinical trial comparing penbutolol (40 mg and greater once a day) and propranolol (40 mg or more twice a day), heart rates of less than 60 beats/min. were recorded at least once in 25% of the patients in the group receiving penbutolol and in 37% of the patients in the propranolol group. Corresponding figures for heart rates of less than 50 beats/min. were 1.2% and 6% respectively. No symptoms associated with bradycardia were reported. | |||
Discontinuations of penbutolol because of adverse reactions have ranged between 2.4% and 6.9% of patients in double-blind, parallel, controlled clinical trials, as compared to 1.8% to 4.1% in the corresponding control groups that were given placebo. The frequency and severity of adverse reactions have not increased during long-term administration of penbutolol. The prevalence of adverse reactions reported from 4 controlled clinical trials (referred to in the table above) as reasons for discontinuation of therapy by>0.5% of the penbutolol group is listed in the table below. | |||
[[File:Penbutolol02.jpg|800px|thumbnail|left|This image is provided by the National Library of Medicine.]] | |||
{{clr}} | |||
: ( | =====Potential Adverse Effects===== | ||
In addition, certain adverse effects not listed above have been reported with other ß-blocking agents and should also be considered as potential adverse effects of penbutolol. | |||
* '''Central Nervous System''': Reversible mental depression progressing to[[ catatonia]] (an acute syndrome characterized by disorientation for time and place), short-term [[memory loss]], [[emotional lability]], slightly clouded sensorium, and decreased performance (neuropsychometrics). | |||
* '''Cardiovascular''': Intensification of [[AV block]]. | |||
* '''Allergic''': [[Erythematous rash]], fever combined with aching and [[sore throat]], [[laryngospasm]], and respiratory distress. | |||
* '''Hematologic''': [[Agranulocytosis]], nonthrombocytopenic and thrombocytopenic [[purpura]]. | |||
* '''Gastrointestinal''': [[Mesenteric arterial thrombosis]] and [[ischemic colitis]]. | |||
* '''Miscellaneous''': Reversible alopecia and Peyronie’s disease. The oculomucocutaneous syndrome associated with the ß-blocker practolol has not been reported with penbutolol during investigational use and extensive foreign clinical experience. | |||
|drugInteractions=* Drug 1 | |drugInteractions=* Drug 1 | ||
* Drug 2 | * Drug 2 | ||
Revision as of 18:35, 11 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Penbutolol is a beta-adrenergic blocker that is FDA approved for the treatment of hypertension. Common adverse reactions include nausea, dizziness, headache, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
The usual starting and maintenance dose of penbutolol, used alone or in combination with other antihypertensive agents, such as thiazide-type diuretics, is 20 mg given once daily.
Doses of 40 mg and 80 mg have been well-tolerated but have not been shown to give a greater antihypertensive effect. The full effect of a 20- or 40-mg dose is seen by the end of 2 weeks. A dose of 10 mg also lowers blood pressure, but the full effect is not seen for 4 to 6 weeks.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Penbutolol in adult patients.
Non–Guideline-Supported Use
Angina Pectoris
- Dosing Information
- 40 mg/day.[1]
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Penbutolol FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Penbutolol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Penbutolol in pediatric patients.
Contraindications
- Cardiogenic shock
- Sinus bradycardia
- Second degree heart block
- Third degree heart block
- Bronchial asthma
- Hypersensitivity to penbutolol
Warnings
Cardiac Failure
Sympathetic stimulation may be essential for supporting circulatory function in patients with heart failure, and its inhibition by ß-adrenergic receptor blockade may precipitate more severe failure. Although ß-blockers should be avoided in overt congestive heart failure, penbutolol can, if necessary, be used with caution in patients with a history of cardiac failure who are well compensated, on treatment with vasodilators, digitalis and/or diuretics. Both digitalis and penbutolol slow AV conduction. Beta-adrenergic receptor antagonists do not inhibit the inotropic action of digitalis on heart muscle. If cardiac failure persists, treatment with penbutolol should be discontinued.
Patients Without History of Cardiac Failure
Continued depression of the myocardium with ß-blocking agents over a period of time can, in some cases, lead to cardiac failure. At the first evidence of heart failure, patients receiving penbutolol should be given appropriate treatment, and the response should be closely observed. If cardiac failure continues despite adequate intervention with appropriate drugs, penbutolol should be withdrawn (gradually, if possible).
Exacerbation of Ischemic Heart Disease Following Abrupt Withdrawal
Hypersensitivity to catecholamines has been observed in patients who were withdrawn from therapy with ß-blocking agents; exacerbation of angina and, in some cases, myocardial infarction have occurred after abrupt discontinuation of such therapy. When discontinuing penbutolol, particularly in patients with ischemic heart disease, the dosage should be reduced gradually over a period of 1 to 2 weeks and the patient should be monitored carefully. If angina becomes more pronounced or acute coronary insufficiency develops, administration of penbutolol should be reinstated promptly, at least on a temporary basis, and appropriate measures should be taken for the management of unstable angina. Patients should be warned against interruption or discontinuation of therapy without the physician’s advice. Because coronary artery disease is common and may not be recognized, it may not be prudent to discontinue penbutolol abruptly, even in patients who are being treated only for hypertension.
Nonallergic Bronchospasm (eg, chronic bronchitis,emphysema)
Penbutolol is contraindicated in bronchial asthma. In general, patients with bronchospastic diseases should not receive ß-blockers. Penbutolol should be administered with caution because it may block bronchodilation produced by endogenous catecholamine stimulation of ß-2 receptors.
Major Surgery
Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery; however, the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
Diabetes Mellitus and Hypoglycemia
Beta-adrenergic receptor blockade may prevent the appearance of signs and symptoms of acute hypoglycemia, such as tachycardia and blood pressure changes. This is especially important in patients with labile diabetes. Beta-blockade also reduces the release of insulin in response to hyperglycemia; therefore, it may be necessary to adjust the dose of hypoglycemic drugs. Beta-adrenergic blockade may also impair the homeostatic response to hypoglycemia; in that event, the spontaneous recovery from hypoglycemia may be delayed during treatment with ß-adrenergic receptor antagonists.
Thyrotoxicosis
Beta-adrenergic blockade may mask certain clinical signs (eg, tachycardia) of hyperthyroidism. Patients suspected of developing thyrotoxicosis should be managed carefully to avoid abrupt withdrawal of ß-adrenergic receptor blockers that might precipitate a thyroid storm.
Adverse Reactions
Clinical Trials Experience
Penbutolol is usually well tolerated in properly selected patients. Most adverse effects observed during clinical trials have been mild and reversible.
Table 1 lists the adverse reactions reported from 4 controlled studies conducted in the United States involving once-a-day administration of penbutolol (at doses ranging from 10 to 120 mg) as monotherapy or in combination with hydrochlorothiazide. Penbutolol doses above 40 mg/day are not, however, recommended. The table includes only those events where the prevalence rate in the penbutolol group was at least 1.5%, or where the reaction is of particular interest.
Over a dose range from 10 to 40 mg, once a day, fatigue, nausea, and sexual impotence occurred at a greater frequency as the dose was increased.
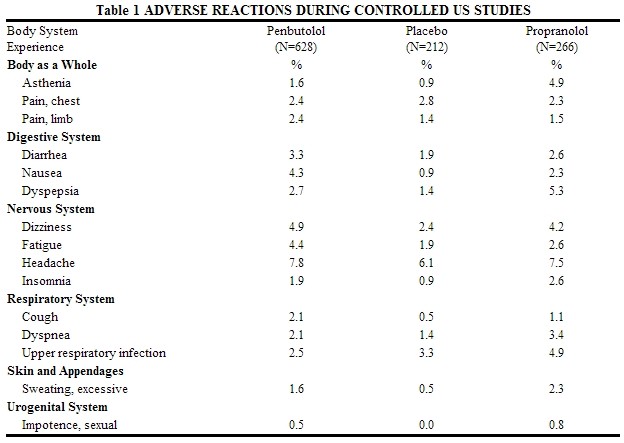
In a double-blind clinical trial comparing In a double-blind clinical trial comparing penbutolol (40 mg and greater once a day) and propranolol (40 mg or more twice a day), heart rates of less than 60 beats/min. were recorded at least once in 25% of the patients in the group receiving penbutolol and in 37% of the patients in the propranolol group. Corresponding figures for heart rates of less than 50 beats/min. were 1.2% and 6% respectively. No symptoms associated with bradycardia were reported.
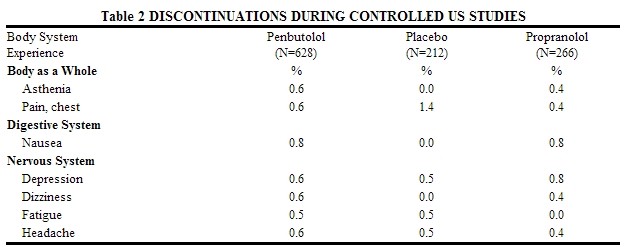
Discontinuations of penbutolol because of adverse reactions have ranged between 2.4% and 6.9% of patients in double-blind, parallel, controlled clinical trials, as compared to 1.8% to 4.1% in the corresponding control groups that were given placebo. The frequency and severity of adverse reactions have not increased during long-term administration of penbutolol. The prevalence of adverse reactions reported from 4 controlled clinical trials (referred to in the table above) as reasons for discontinuation of therapy by>0.5% of the penbutolol group is listed in the table below.
Potential Adverse Effects
In addition, certain adverse effects not listed above have been reported with other ß-blocking agents and should also be considered as potential adverse effects of penbutolol.
- Central Nervous System: Reversible mental depression progressing tocatatonia (an acute syndrome characterized by disorientation for time and place), short-term memory loss, emotional lability, slightly clouded sensorium, and decreased performance (neuropsychometrics).
- Cardiovascular: Intensification of AV block.
- Allergic: Erythematous rash, fever combined with aching and sore throat, laryngospasm, and respiratory distress.
- Hematologic: Agranulocytosis, nonthrombocytopenic and thrombocytopenic purpura.
- Gastrointestinal: Mesenteric arterial thrombosis and ischemic colitis.
- Miscellaneous: Reversible alopecia and Peyronie’s disease. The oculomucocutaneous syndrome associated with the ß-blocker practolol has not been reported with penbutolol during investigational use and extensive foreign clinical experience.
Postmarketing Experience
There is limited information regarding Penbutolol Postmarketing Experience in the drug label.
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
(Description)
Labor and Delivery
(Description)
Nursing Mothers
(Description)
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Solution
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
Penbutolol
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Penbutolol Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Penbutolol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Penbutolol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Penbutolol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Penbutolol Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Bowles MJ, Khurmi NS, Bala Subramanian V, Raftery EB (1984). "Efficacy of once daily penbutolol in chronic stable angina. An objective comparison with long-acting propranolol". Int J Cardiol. 5 (2): 131–42. PMID 6365803.