Paroxysmal nocturnal hemoglobinuria
| Paroxysmal nocturnal hemoglobinuria | |
| ICD-10 | D59.5 |
|---|---|
| ICD-9 | 283.2 |
| OMIM | 311770 |
| DiseasesDB | 9688 |
| eMedicine | med/2696 |
| MeSH | D006457 |
Template:Search infobox Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired, potentially life-threatening disease of the blood characterised by hemolytic anemia, thrombosis and red urine due to breakdown of red blood cells. PNH is the only hemolytic anemia caused by an acquired intrinsic defect in the cell membrane.
History
The first description of paroxysmal hemoglobinuria was by the German physician Paul Strübing (1852).[1][2] A more detailed description was made by Dr Ettore Marchiafava and Dr Alessio Nazari in 1911,[3] with further elaborations by Marchiafava in 1928[4] and Dr Ferdinando Micheli in 1931.[5]
Classification
PNH is classified:
- Classic PNH. Evidence of PNH in the absence of another bone marrow disorder.
- PNH in the setting of another specified bone marrow disorder.
- Subclinical PNH. PNH abnormalities on flow cytometry without signs of hemolysis.
Pathophysiology
All cells have proteins attached to their membranes and they are responsible for performing a vast array of functions. There are several ways for proteins to be attached to a cell membrane. PNH occurs as a result of a defect in one of these mechanisms.
A molecule called PIGA (phosphatidylinositol glycan A) is needed to make a cell membrane anchor for proteins called GPI (glycosylphosphatidylinositol).[6] The gene that codes for PIGA is inherited in an X-linked fashion, which means that only one active copy of the gene for PIGA may exist. If a mutation occurs in this gene then PIGA may be defective, which leads to a defect in the GPI anchor. When this mutation occurs in a bone marrow stem cell (which are used to make red blood cells as well as white blood cells and platelets), all of the cells it produces will also have the defect. Several of the proteins that anchor to GPI on the cell membrane are used to protect the cell from destruction by the complement system. The complement system is part of the immune system and helps to destroy invading microorganisms. Without the proteins that protect them from complement, red blood cells are destroyed. The main proteins which carry out this function are CD16, CD55 and CD59 (CD is an acronym for cluster of differentiation).
The increased destruction of red blood cells results in anemia. The increased rate of thrombosis is due to dysfunction of platelets. They are also made by the bone marrow stem cells and will have the same GPI anchor defect as the red blood cells. The proteins which use this anchor are needed for platelets to clot properly, and their absence leads to a hypercoagulable state.
Signs and symptoms
Quite paradoxically, the destruction of red blood cells (hemolysis) is neither paroxysmal nor nocturnal the majority of the time (this constellation of symptoms is seen in only 25% of patients). On-going hemolysis is a more common characteristic.
A common finding in PNH is the presence of breakdown products of RBCs (hemoglobin and hemosiderin) in the urine.
An inconsistent, but potentially life-threatening, complication of PNH is the development of clot in the veins (venous thrombosis). These clots (thrombi) are often found in the hepatic (causing Budd-Chiari syndrome), portal (causing portal vein thrombosis), and cerebral veins (causing cerebral venous thrombosis).
Many patients with bone marrow failure (aplastic anemia) develop PNH (10-33%). Aplastic anemia can be caused by an attack by the immune system against the bone marrow. For this reason, drugs that suppress the immune system are being researched as a therapy for PNH.[7] [8]
Diagnosis
A sugar or sucrose lysis test, in which a patient's red blood cells are placed in low ionic strength solution and observed for hemolysis, is used for screening. A more specific test for PNH, called Ham's acid hemolysis test, is performed if the sugar test is positive for hemolysis.[9]
Modern methods include flow cytometry for CD55, CD16 and CD59 on white and red blood cells. [10]Dependent on the presence of these molecules on the cell surface, they are classified as type I, II or III PNH cells.
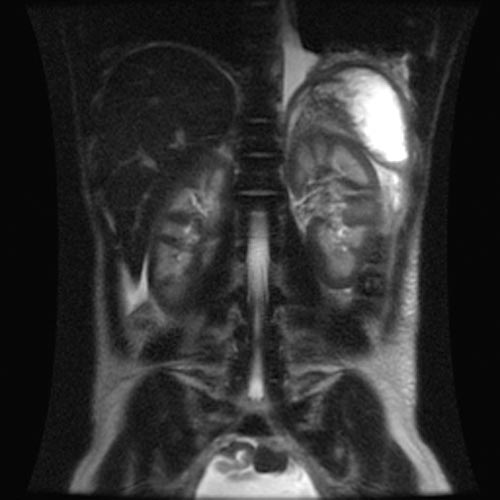
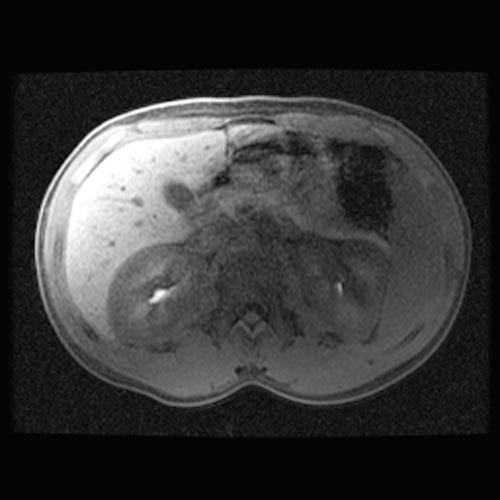
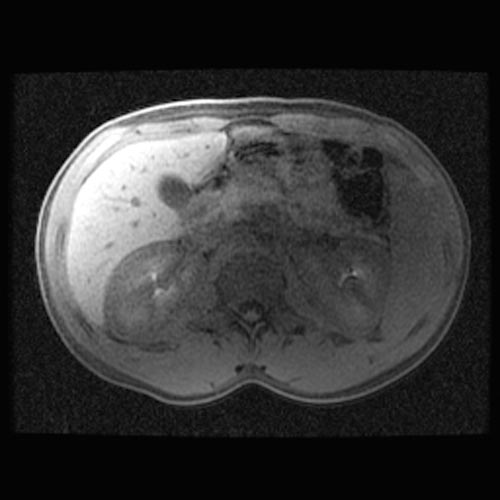
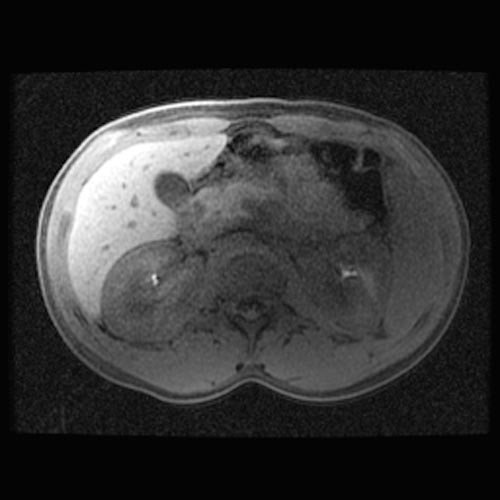
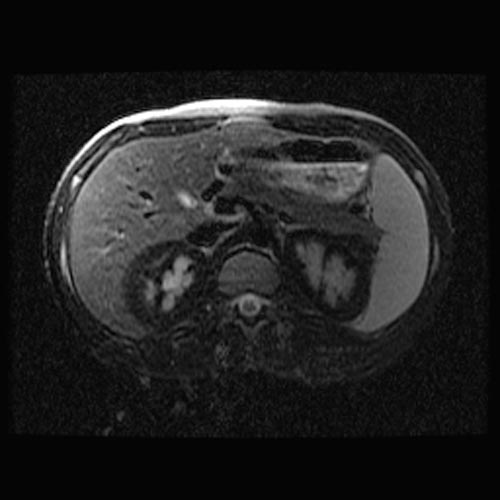
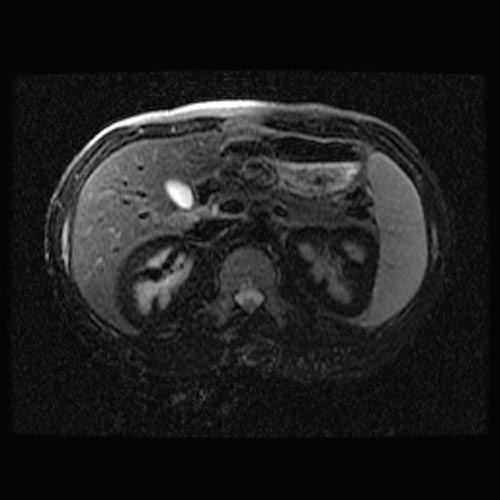
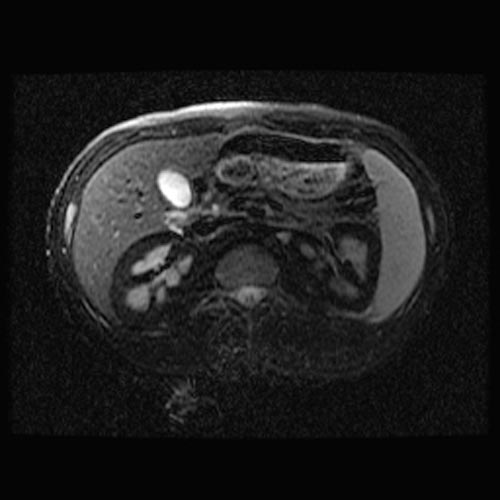
MRI
- Renal cortical signal intensity loss (hemosiderin accumulates in the renal cortex when intravascular hemolysis results in the direct release of hemoglobin into the plasma).
- Venous thrombosis.
- Liver and spleen are usually of normal signal intensity in paroxysmal nocturnal hemoglobinuria, unless repeated transfusions have resulted in hepatic and splenic signal intensity loss owing to transfusional siderosis.
(Images shown below are courtesy of RadsWiki)
Treatment
Long-term
PNH is a chronic condition. In patients who have only a small clone and few problems, monitoring of the flow cytometry every six months gives information on the severity and risk of potential complications. Given the high risk of thrombosis in PNH, preventative treatment with warfarin decreases the risk of thrombosis in those with a large clone (50% of white blood cells type III).[11][12]
Episodes of thrombosis are treated as they would in other patients, but given that PNH is a persisting underlying cause it is likely that treatment with warfarin or similar drugs needs to be continued long-term after an episode of thrombosis.[11]
Acute attacks
There is disagreement as to whether steroids (such as prednisolone) can decrease the severity of hemolytic crises. Transfusion therapy may be needed; in addition to correcting significant anemia this suppresses the production of PNH cells by the bone marrow, and indirectly the severity of the hemolysis. Iron deficiency develops with time, due to losses in urine, and may have to be treated if present. Iron therapy can result in more hemolysis as more PNH cells are produced.[11]
A new monoclonal antibody, eculizumab, protects blood cells against immune destruction by inhibiting the complement system. It has been shown to reduce the need for blood transfusion in patients with significant hemolysis.[13]
References
- ↑ Strübing P. Paroxysmale Hämoglobinurie. Dtsch Med Wochenschr 1882;8:1-3 and 17-21.
- ↑ Whonamedit entry
- ↑ Marchiafava E, Nazari A. Nuovo contributo allo studio degli itteri cronici emolitici. Policlinico [Med] 1911;18:241-254.
- ↑ Marchiafava E. Anemia emolitica con emosiderinuria perpetua. Policlinico [Med] 1928;35:105-117.
- ↑ Micheli F. Uno caso di anemia emolitica con emosiderinuria perpetua. G Accad Med Torino 1931;13:148.
- ↑ Hu R, Mukhina GL, Piantadosi S, Barber JP, Jones RJ, Brodsky RA. PIG-A mutations in normal hematopoiesis. Blood 2005;105:3848-54. PMID 15687243.
- ↑ Sacher, Ronald A. and Richard A. McPherson. "Wildman's Clinical Interpretation of Laboratory Tests, 11th edition."
- ↑ Kumar, Vinay, Abu Abbas, and Nelson Fausto. "Robbins and Cotran Pathologic Basis of Disease, 7th edition."
- ↑ Ham TH. Chronic haemolytic anaemia with paroxysmal nocturnal haemoglobinuria: study of the mechanism of haemolysis in relation to acid-base equilibtium. N Engl J Med 1937;217:915-918.
- ↑ Parker C, Omine M, Richards S, Nishimura J, Bessler M, Ware R, Hillmen P, Luzzatto L, Young N, Kinoshita T, Rosse W, Socie G, International PNH Interest Group. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood 2005;106:3699-709. PMID 16051736.
- ↑ 11.0 11.1 11.2
- ↑ Hall C, Richards S, Hillmen P (2003). "Primary prophylaxis with warfarin prevents thrombosis in paroxysmal nocturnal hemoglobinuria (PNH)". Blood. 102 (10): 3587–91. doi:10.1182/blood-2003-01-0009. PMID 12893760. Unknown parameter
|month=ignored (help) - ↑ Hillmen P, Hall C, Marsh JC; et al. (2004). "Effect of eculizumab on hemolysis and transfusion requirements in patients with paroxysmal nocturnal hemoglobinuria". N. Engl. J. Med. 350 (6): 552–9. doi:10.1056/NEJMoa031688. PMID 14762182.
Additional Resources
- Ross L. Titton, and Fergus V. Coakley. Case 51: Paroxysmal Nocturnal Hemoglobinuria with Thrombotic Budd-Chiari Syndrome and Renal Cortical Hemosiderin. Radiology 2002 225: 67-70.
External links
- Aplastic Anemia & MDS International Foundation
- PNH Support Group
- PNH Research and Support Foundation
- Goldminer: Paroxysmal nocturnal hemoglobinuria
Template:Hematology Template:SIB
de:Paroxysmale nächtliche Hämoglobinurie it:Emoglobinuria parossistica notturna nl:Paroxysmale nocturnale hemoglobinurie sr:Пароксизмална ноћна хемоглобинурија