Parkinson's disease pathophysiology: Difference between revisions
No edit summary |
Ahmed Younes (talk | contribs) No edit summary |
||
| Line 1: | Line 1: | ||
<div style="-webkit-user-select: none;"> | |||
{|class="infobox" style="position: fixed; top: 65%; right: 10px; margin: 0 0 0 0; border: 0; float: right; | |||
|- | |||
| {{#ev:youtube|https://https://www.youtube.com/watch?v=VIEUEV9wlyI|350}} | |||
|- | |||
|} | |||
__NOTOC__ | __NOTOC__ | ||
{{Parkinson's disease}} | {{Parkinson's disease}} | ||
Revision as of 15:42, 10 July 2017
| https://https://www.youtube.com/watch?v=VIEUEV9wlyI%7C350}} |
|
Parkinson's disease Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Parkinson's disease pathophysiology On the Web |
|
American Roentgen Ray Society Images of Parkinson's disease pathophysiology |
|
Risk calculators and risk factors for Parkinson's disease pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Pathophysiology
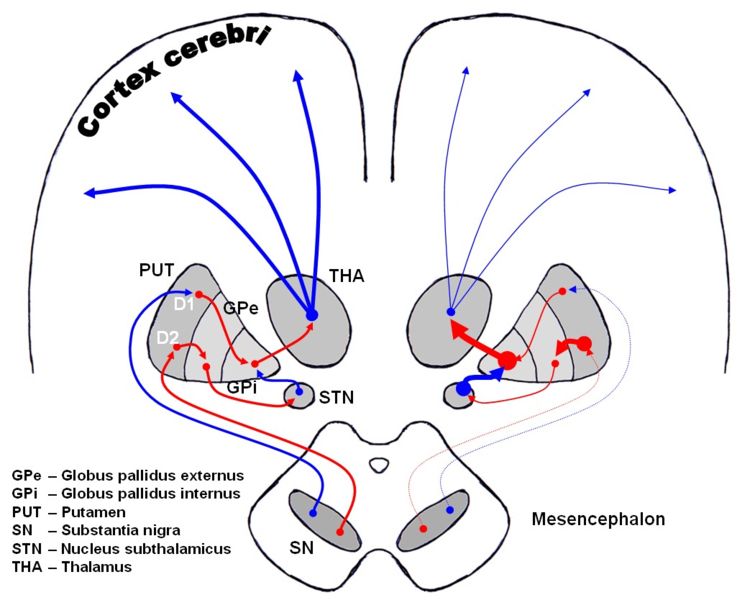
In the brain the direct pathway facilitates movement and the indirect pathway inhibits movement, thus the loss of these cells leads to a hypokinetic movement disorder. The lack of dopamine results in increased inhibition of the ventral lateral nucleus of the thalamus, which sends excitatory projections to the motor cortex, thus leading to hypokinesia.
There are four major dopamine pathways in the brain; the nigrostriatal pathway, referred to above, mediates movement and is the most conspicuously affected in early Parkinson's disease. The other pathways are the mesocortical, the mesolimbic, and the tuberoinfundibular. These pathways are associated with, respectively: volition and emotional responsiveness; desire, initiative, and reward; and sensory processes and maternal behavior. Disruption of dopamine along the non-striatal pathways likely explains much of the neuropsychiatric pathology associated with Parkinson's disease.
The mechanism by which the brain cells in Parkinson's are lost may consist of an abnormal accumulation of the protein alpha-synuclein bound to ubiquitin in the damaged cells. The alpha-synuclein-ubiquitin complex cannot be directed to the proteosome. This protein accumulation forms proteinaceous cytoplasmic inclusions called Lewy bodies. Latest research on pathogenesis of disease has shown that the death of dopaminergic neurons by alpha-synuclein is due to a defect in the machinery that transports proteins between two major cellular organelles — the endoplasmic reticulum (ER) and the Golgi apparatus. Certain proteins like Rab1 may reverse this defect caused by alpha-synuclein in animal models.[1]
Excessive accumulations of iron, which are toxic to nerve cells, are also typically observed in conjunction with the protein inclusions. Iron and other transition metals such as copper bind to neuromelanin in the affected neurons of the substantia nigra. So, neuromelanin may be acting as a protective agent. Alternately, neuromelanin (an electronically active semiconductive polymer) may play some other role in neurons.[2] That is, coincidental excessive accumulation of transition metals, etc. on neuromelanin may figure in the differential dropout of pigmented neurons in Parkinsonism. The most likely mechanism is generation of reactive oxygen species.[3]
Iron induces aggregation of synuclein by oxidative mechanisms.[4] Similarly, dopamine and the byproducts of dopamine production enhance alpha-synuclein aggregation. The precise mechanism whereby such aggregates of alpha-synuclein damage the cells is not known. The aggregates may be merely a normal reaction by the cells as part of their effort to correct a different, as-yet unknown, insult. Based on this mechanistic hypothesis, a transgenic mouse model of Parkinson's has been generated by introduction of human wild-type α-synuclein into the mouse genome under control of the platelet-derived-growth factor-β promoter.[5]
-
Dopaminergic pathways of the human brain in normal condition (left) and Parkinson's disease (right). Red Arrows indicate suppression of the target, blue arrows indicate stimulation of target structure.
The symptoms of Parkinson's disease result from the loss of pigmented dopamine-secreting (dopaminergic) cells, secreted by the same cells, in the pars compacta region of the substantia nigra (literally "black substance"). These neurons project to the striatum and their loss leads to alterations in the activity of the neural circuits within the basal ganglia that regulate movement, in essence an inhibition of the direct pathway and excitation of the indirect pathway.
Genetic
In recent years, a number of specific genetic mutations causing Parkinson's disease have been discovered, including in certain populations (Contursi, Italy). These account for a small minority of cases of Parkinson's disease. Somebody who has Parkinson's disease is more likely to have relatives that also have Parkinson's disease. However, this does not mean that the disorder has been passed on genetically.
Genetic forms that have been identified include:
- external links in this section are to OMIM
| Type | OMIM | Locus | Details |
| PARK1 | OMIM #168601 | 4q21 | caused by mutations in the SNCA gene, which codes for the protein alpha-synuclein. PARK1 causes autosomal dominant Parkinson disease. So-called PARK4 (OMIM #605543) is probably caused by triplication of SNCA.[6] |
| PARK2 | OMIM *602544 | 6q25.2-q27 | caused by mutations in protein parkin. Parkin mutations may be one of the most common known genetic causes of early-onset Parkinson disease. In one study, of patients with onset of Parkinson disease prior to age 40 (10% of all PD patients), 18% had parkin mutations, with 5% homozygous mutations.[7] Patients with an autosomal recessive family history of parkinsonism are much more likely to carry parkin mutations if age at onset is less than 20 (80% vs. 28% with onset over age 40).[8]Patients with parkin mutations (PARK2) do not have Lewy bodies. Such patients develop a syndrome that closely resembles the sporadic form of PD; however, they tend to develop symptoms at a much younger age. |
| PARK3 | OMIM %602404 | 2p13 | autosomal dominant, only described in a few kindreds. |
| PARK5 | OMIM +191342 | 4p14 | caused by mutations in the UCHL1 gene which codes for the protein ubiquitin carboxy-terminal hydrolase L1 |
| PARK6 | OMIM #605909 | 1p36 | caused by mutations in PINK1 (OMIM *608309) which codes for the protein PTEN-induced putative kinase 1. |
| PARK7 | OMIM #606324 | 1p36 | caused by mutations in DJ-1 (OMIM 602533) |
| PARK8 | OMIM #607060 | 12q12 | caused by mutations in LRRK2 which codes for the protein dardarin. In vitro, mutant LRRK2 causes protein aggregation and cell death, possibly through an interaction with parkin.[9] LRRK2 mutations, of which the most common is G2019S, cause autosomal dominant Parkinson disease, with a penetrance of nearly 100% by age 80.[10] G2019S is the most common known genetic cause of Parkinson disease, found in 1-6% of U.S. and European PD patients.[11] It is especially common in Ashkenazi Jewish patients, with a prevalence of 29.7% in familial cases and 13.3% in sporadic.[12] |
| PARK9 | OMIM #606693 | 1p36 | Caused by mutations in the ATP13A2 gene, and also known as Kufor-Rakeb Syndrome. PARK9 may be allelic to PARK6. |
| PARK10 | OMIM %606852 | 1p | - |
| PARK11 | OMIM %607688 | 2q36-37 | However, this gene locus has conflicting data, and may not have significance. |
| PARK12 | OMIM %300557 | Xq21-q25 | - |
| PARK13 | OMIM #610297 | 2p12 | Caused by mutations in the HTRA2 (HtrA serine peptidase 2) gene. |
Toxins
One theory holds that the disease may result in many or even most cases from the combination of a genetically determined vulnerability to environmental toxins along with exposure to those toxins.[13] This hypothesis is consistent with the fact that Parkinson's disease is not distributed homogeneously throughout the population: rather, its incidence varies geographically. It would appear that incidence varies by time as well, for although the later stages of untreated PD are distinct and readily recognizable, the disease was not remarked upon until the beginnings of the Industrial Revolution, and not long thereafter become a common observation in clinical practice. The toxins most strongly suspected at present are certain pesticides and transition-series metals such as manganese or iron, especially those that generate reactive oxygen species,[3][14] and or bind to neuromelanin, as originally suggested by G.C. Cotzias.[15][16]. In the Cancer Prevention Study II Nutrition Cohort, a longitudinal investigation, individuals who were exposed to pesticides had a 70% higher incidence of PD than individuals who were not exposed[17].
MPTP is used as a model for Parkinson's as it can rapidly induce parkinsonian symptoms in human beings and other animals, of any age. MPTP was notorious for a string of Parkinson's disease cases in California in 1982 when it contaminated the illicit production of the synthetic opiate MPPP. Its toxicity likely comes from generation of reactive oxygen species through tyrosine hydroxylation.[18]
Other toxin-based models employ PCBs,[19] paraquat[20] (a herbicide) in combination with maneb (a fungicide)[21] rotenone[22] (an insecticide), and specific organochlorine pesticides including dieldrin[23] and lindane.[24] Numerous studies have found an increase in PD in persons who consume rural well water; researchers theorize that water consumption is a proxy measure of pesticide exposure. In agreement with this hypothesis are studies which have found a dose-dependent an increase in PD in persons exposed to agricultural chemicals.
Head trauma
Past episodes of head trauma are reported more frequently by sufferers than by others in the population.[25][26][27] A methodologically strong recent study[25] found that those who have experienced a head injury are four times more likely to develop Parkinson’s disease than those who have never suffered a head injury. The risk of developing Parkinson’s increases eightfold for patients who have had head trauma requiring hospitalization, and it increases 11-fold for patients who have experienced severe head injury. The authors comment that since head trauma is a rare event, the contribution to PD incidence is slight. They express further concern that their results may be biased by recall, i.e., the PD patients because they reflect upon the causes of their illness, may remember head trauma better than the non-ill control subjects. These limitations were overcome recently by Tanner and colleagues,[28] who found a similar risk of 3.8, with increasing risk associated with more severe injury and hospitalization.
Drug-induced
Antipsychotics, which are used to treat schizophrenia and psychosis, can induce the symptoms of Parkinson's disease (or parkinsonism) by lowering dopaminergic activity. Due to feedback inhibition, L-dopa can also eventually cause the symptoms of Parkinson's disease that it initially relieves. Dopamine agonists can also eventually contribute to Parkinson's disease symptoms by decreasing the sensitivity of dopamine receptors.
Associated Disorders
There are other disorders that are called Parkinson-plus diseases. These include:
- Multiple system atrophy (MSA)
- Progressive supranuclear palsy (PSP)
- Corticobasal degeneration (CBD)
Some people include dementia with Lewy bodies (DLB) as one of the 'Parkinson-plus' syndromes. Although idiopathic Parkinson's disease patients also have Lewy bodies in their brain tissue, the distribution is denser and more widespread in DLB. Even so, the relationship between Parkinson disease, Parkinson disease with dementia (PDD) and dementia with Lewy bodies (DLB) might be most accurately conceptualized as a spectrum, with a discrete area of overlap between each of the three disorders. The natural history and role of Lewy bodies is very little understood.
Patients often begin with typical Parkinson's disease symptoms which persist for some years; these Parkinson-plus diseases can only be diagnosed when other symptoms become apparent with the passage of time. These Parkinson-plus diseases usually progress more quickly than typical ideopathic Parkinson disease. The usual anti-Parkinson's medications are typically either less effective or not effective at all in controlling symptoms; patients may be exquisitely sensitive to neuroleptic medications like haloperidol. Additionally, the cholinesterase inhibiting medications have shown preliminary efficacy in treating the cognitive, psychiatric, and behavioral aspects of the disease, so correct differential diagnosis is important.
Wilson's disease (hereditary copper accumulation) may present with parkinsonistic features; young patients presenting with parkinsonism may be screened for this rare condition. Essential tremor is often mistaken for Parkinson's disease but usually lacks all features besides tremor.
Torsion dystonia is another disease related to Parkinson's disease.
References
- ↑ "Parkinson's Disease Mechanism Discovered," HHMI Research News June 22, 2006.
- ↑ McGinness J, Corry P, Proctor P (1974). "Amorphous semiconductor switching in melanins" (Reprint). Science. 183 (127): 853–5. PMID 4359339.
- ↑ 3.0 3.1 Jenner P (1998). "Oxidative mechanisms in nigral cell death in Parkinson's disease". Mov Disord. 13 Suppl 1: 24–34. PMID 9613715.
- ↑ Kaur D, Andersen J (2002). "Ironing out Parkinson's disease: is therapeutic treatment with iron chelators a real possibility?" (PDF). Aging Cell. 1 (1): 17–21. PMID 12882349.
- ↑ Masliah E, Rockenstein E, Veinbergs I; et al. (2000). "Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders". Science. 287 (5456): 1265–9. PMID 10678833.
- ↑ Singleton AB, Farrer M, Johnson J; et al. (2003). "alpha-Synuclein locus triplication causes Parkinson's disease". Science. 302 (5646): 841. doi:10.1126/science.1090278. PMID 14593171.
- ↑ Poorkaj P; et al. (2004). "parkin mutation analysis in clinic patients with early-onset Parkinson's disease". American Journal of Medical Genetics Part A. 129A (1): 44&ndash, 50.
- ↑ Ebba Lohmann; et al. (2003). "How much phenotypic variation can be attributed to parkin genotype?". Annals of Neurology. 54 (2): 176&ndash, 185. PMID 12891670.
- ↑ Smith WW; et al. (2005). "Leucine-rich repeat kinase 2 (LRRK2) interacts with parkin, and mutant LRRK2 induces neuronal degeneration". Proceedings of the National Academy of Sciences of the United States of America. 102 (51): 18676&ndash, 18681. PMID 16352719.
- ↑ Kachergus J, Mata IF, Hulihan M; et al. (2005). "Identification of a novel LRRK2 mutation linked to autosomal dominant parkinsonism: evidence of a common founder across European populations". Am. J. Hum. Genet. 76 (4): 672–80. doi:10.1086/429256. PMID 15726496.
- ↑ Brice A (2005). "Genetics of Parkinson's disease: LRRK2 on the rise". Brain. 128 (Pt 12): 2760–2. doi:10.1093/brain/awh676. PMID 16311269.
- ↑ Ozelius L, Senthil G, Saunders-Pullman R; et al. (2006). "LRRK2 G2019S as a cause of Parkinson's disease in Ashkenazi Jews". N Engl J Med. 354 (4): 424–5. PMID 16436782.
- ↑ Di Monte DA, Lavasani M, Manning-Bog AB (2002). "Environmental factors in Parkinson's disease". Neurotoxicology. 23 (4–5): 487–502. PMID 12428721.
- ↑ Chiueh CC, Andoh T, Lai AR, Lai E, Krishna G (2000). "Neuroprotective strategies in Parkinson's disease: protection against progressive nigral damage induced by free radicals". Neurotoxicity research. 2 (2–3): 293–310. PMID 16787846.
- ↑ Cotzias G (1966). "Manganese, melanins and the extrapyramidal system". J Neurosurg. 24 (1): Suppl:170-80. PMID 4955707.
- ↑ Barbeau A (1984). "Manganese and extrapyramidal disorders (a critical review and tribute to Dr. George C. Cotzias)". Neurotoxicology. 5 (1): 13–35. PMID 6538948.
- ↑ Ascherio A, Chen H, Weisskopf M; et al. (2006). "Pesticide exposure and risk for Parkinson's disease". Ann Neurol. 60 (2): 197–203. PMID 16802290.
- ↑ Chiueh C, Wu R, Mohanakumar K, Sternberger L, Krishna G, Obata T, Murphy D (1994). "In vivo generation of hydroxyl radicals and MPTP-induced dopaminergic toxicity in the basal ganglia". Ann N Y Acad Sci. 738: 25–36. PMID 7832434.
- ↑ Orr, Leslie (February 10, 2005). "PCBs, fungicide open brain cells to Parkinson's assault". Medical News Today.
- ↑ Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA (2002). "The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein". J. Biol. Chem. 277 (3): 1641–4. doi:10.1074/jbc.C100560200. PMID 11707429.
- ↑ Thiruchelvam M, Richfield EK, Baggs RB, Tank AW, Cory-Slechta DA (2000). "The nigrostriatal dopaminergic system as a preferential target of repeated exposures to combined paraquat and maneb: implications for Parkinson's disease". J. Neurosci. 20 (24): 9207–14. PMID 11124998.
- ↑ Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000). "Chronic systemic pesticide exposure reproduces features of Parkinson's disease". Nat. Neurosci. 3 (12): 1301–6. doi:10.1038/81834. PMID 11100151.
- ↑ Kitazawa M, Anantharam V, Kanthasamy AG (2001). "Dieldrin-induced oxidative stress and neurochemical changes contribute to apoptopic cell death in dopaminergic cells". Free Radic. Biol. Med. 31 (11): 1473–85. PMID 11728820.
- ↑ Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D (2000). "Organochlorine insecticides in substantia nigra in Parkinson's disease". J. Toxicol. Environ. Health Part A. 59 (4): 229–34. PMID 10706031.
- ↑ 25.0 25.1 Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, Ahlskog JE, Rocca WA (2003). "Head trauma preceding PD: a case-control study". Neurology. 60 (10): 1610–5. PMID 12771250.
- ↑ Stern M, Dulaney E, Gruber SB; et al. (1991). "The epidemiology of Parkinson's disease. A case-control study of young-onset and old-onset patients". Arch. Neurol. 48 (9): 903–7. PMID url=http://archneur.ama-assn.org/cgi/content/abstract/48/9/903 1953412 url=http://archneur.ama-assn.org/cgi/content/abstract/48/9/903 Check
|pmid=value (help). - ↑ Uryu K, Giasson BI, Longhi L; et al. (2003). "Age-dependent synuclein pathology following traumatic brain injury in mice". Exp. Neurol. 184 (1): 214–24. PMID 14637093.
- ↑ Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW (2006). "Head injury and Parkinson's disease risk in twins". Ann. Neurol. 60 (1): 65–72. doi:10.1002/ana.20882. PMID 16718702.