Multiple endocrine neoplasia type 2 pathophysiology: Difference between revisions
No edit summary |
|||
| (71 intermediate revisions by 7 users not shown) | |||
| Line 2: | Line 2: | ||
{{Multiple endocrine neoplasia type 2}} | {{Multiple endocrine neoplasia type 2}} | ||
{{CMG}}; {{AE}} {{Ammu}} | {{CMG}}; {{AE}} {{Ammu}} | ||
==Overview== | ==Overview== | ||
The progression to multiple endocrine neoplasia type 2 usually involves the [[Genetics|genetic]] [[Mutation|mutations]]. The pathogenesis of multiple endocrine neoplasia type 2 involves a mutation of the ''[[RET gene|RET]]'' [[gene]]. | |||
==Pathogenesis== | ==Pathogenesis== | ||
The common feature among the three subtypes of multiple endocrine neoplasia type 2 is a high propensity to develop [[medullary thyroid carcinoma]]. | |||
===Multiple | ===Multiple Endocrine Neoplasia type 2A=== | ||
* Multiple endocrine neoplasia type 2 (MEN2) (also | * Multiple endocrine neoplasia type 2 (MEN2) generally occur in [[endocrine organ]]s (e.g. [[thyroid]], [[parathyroid]], and [[Adrenal gland|adrenals]]), but may also occur in [[endocrine]] [[tissues]] of organs not classically thought of as [[endocrine]].<ref name="Moline">{{cite journal | author = Moline J, Eng C. | title = Multiple endocrine neoplasia type 2: an overview. | journal = Genet Med. | year=2011 | volume=9 | issue=13 | pages=755–64 | pmid = 21552134 | doi =10.1097/GIM.0b013e318216cc6d}}</ref> | ||
* Although many different types of [[hormone]]-producing [[tumor]]s are associated with multiple endocrine neoplasia type 2, the most common manifestation is a form of [[thyroid]] [[cancer]] called [[thyroid cancer#medullary thyroid cancer (MTC)|medullary thyroid carcinoma]]. This [[tumor]] secretes an inactive [[hormone]] called [[calcitonin]]. <ref name="CoteGrubbs2015">{{cite journal|last1=Cote|first1=Gilbert J.|last2=Grubbs|first2=Elizabeth G.|last3=Hofmann|first3=Marie-Claude|title=Thyroid C-Cell Biology and Oncogenic Transformation|volume=204|year=2015|pages=1–39|issn=0080-0015|doi=10.1007/978-3-319-22542-5_1}}</ref> | |||
* In | *Many people with this disorder also develop [[pheochromocytoma]], which is a [[tumor]] of the [[adrenal gland]]s (located above each [[kidney]]) that can cause dangerously high [[blood pressure]]. In addition, overactivity of the [[parathyroid gland]] ([[hyperparathyroidism]]) occurs in some cases of [[multiple endocrine neoplasia type 2]]. [[Hyperparathyroidism]] disrupts the normal balance of [[calcium]] in the [[blood]], which can lead to [[kidney stones]], thinning of [[bone]]s, [[weakness]], and [[fatigue]].<ref name="KantorovichPacak2010">{{cite journal|last1=Kantorovich|first1=Vitaly|last2=Pacak|first2=Karel|title=Pheochromocytoma and Paraganglioma|volume=182|year=2010|pages=343–373|issn=00796123|doi=10.1016/S0079-6123(10)82015-1}}</ref> | ||
* | * In multiple endocrine neoplasia type 2, primary [[hyperparathyroidism]] occurs in only 10–30% and is usually diagnosed after the third decade of life. It can occur in children but this is rare. It may be the sole clinical manifestation of this syndrome but this is unusual.<ref name="WellsPacini2013">{{cite journal|last1=Wells|first1=Samuel A.|last2=Pacini|first2=Furio|last3=Robinson|first3=Bruce G.|last4=Santoro|first4=Massimo|title=Multiple Endocrine Neoplasia Type 2 and Familial Medullary Thyroid Carcinoma: An Update|journal=The Journal of Clinical Endocrinology & Metabolism|volume=98|issue=8|year=2013|pages=3149–3164|issn=0021-972X|doi=10.1210/jc.2013-1204}}</ref> | ||
* | * Multiple endocrine neoplasia type 2 associates [[medullary thyroid carcinoma]] with [[pheochromocytoma]] in about 20–50% of cases and with primary [[hyperparathyroidism]] in 5–20% of cases.<ref name="AlmeidaStratakis2010">{{cite journal|last1=Almeida|first1=Madson Q.|last2=Stratakis|first2=Constantine A.|title=Solid tumors associated with multiple endocrine neoplasias|journal=Cancer Genetics and Cytogenetics|volume=203|issue=1|year=2010|pages=30–36|issn=01654608|doi=10.1016/j.cancergencyto.2010.09.006}}</ref> | ||
* Other components of the [[disease]] are absent in familial isolated [[medullary thyroid carcinoma]].<ref name="Niccoli-SireConte-Devolx2007">{{cite journal|last1=Niccoli-Sire|first1=P.|last2=Conte-Devolx|first2=B.|title=Néoplasies endocriniennes multiples de type 2|journal=Annales d'Endocrinologie|volume=68|issue=5|year=2007|pages=317–324|issn=00034266|doi=10.1016/j.ando.2007.04.005}}</ref> | |||
===Multiple Endocrine Neoplasia type 2B=== | ===Multiple Endocrine Neoplasia type 2B=== | ||
* Multiple endocrine neoplasia type 2B | * Multiple endocrine neoplasia type 2B is associated with [[medullary thyroid carcinoma]] and [[pheochromocytoma]] as well as with marfanoid habitus and with [[mucosal]] and [[digestive]] [[neurofibromatosis]]. | ||
{| style="border: 0px; font-size: 90%; margin: 3px;" align="center" | |||
|+'''''Clinical features of multiple endocrine neoplasia syndrome''''' | |||
! style="background: #4479BA; width: 120px;" | {{fontcolor|#FFF|Subtype}} | |||
! style="background: #4479BA; width: 550px;" | {{fontcolor|#FFF|Medullary Thyroid Carcinoma}} | |||
! style="background: #4479BA; width: 550px;" | {{fontcolor|#FFF|Pheochromocytoma}} | |||
! style="background: #4479BA; width: 550px;" | {{fontcolor|#FFF|Parathyroid Disease}} | |||
|- | |||
! style="background: #F5F5F5;" | Multiple endocrine neoplasia type 2A | |||
! style="background: #F5F5F5;" | 95% | |||
! style="background: #F5F5F5;" | 50% | |||
! style="background: #F5F5F5;" | 20% to 30% | |||
|- | |||
! style="background: #F5F5F5;" | Multiple endocrine neoplasia type 2B | |||
! style="background: #F5F5F5;" | 100% | |||
! style="background: #F5F5F5;" | 50% | |||
! style="background: #F5F5F5;" | Uncommon | |||
|- | |||
! style="background: #F5F5F5;" | Familial medullary thyroid carcinoma | |||
! style="background: #F5F5F5;" | 100% | |||
! style="background: #F5F5F5;" | 0% | |||
! style="background: #F5F5F5;" | 0% | |||
|} | |||
==Genetics== | ===Genetics=== | ||
[[Image:autodominant2.jpg|thumb| | [[Image:autodominant2.jpg|thumb|center|500px|Autosomal dominent pattern of inheritance - By nih.gov, Public Domain, https://commons.wikimedia.org/w/index.php?curid=1098109]] | ||
* Most cases of multiple endocrine neoplasia type 2 are inherited in an [[autosomal dominant]] pattern, which means affected people may have affected siblings and relatives in successive generations (such as parents and children). An affected person usually has one parent with the condition. Some cases, however, result from new | * Most cases of multiple endocrine neoplasia type 2 are inherited in an [[autosomal dominant]] pattern, which means affected people may have affected siblings and relatives in successive generations (such as parents and children). An affected person usually has one parent with the condition. Some cases, however, result from new [[mutation]]s in the [[RET proto-oncogene|''RET'' proto-oncogene]]. These cases occur in people with no history of the disorder in their family. | ||
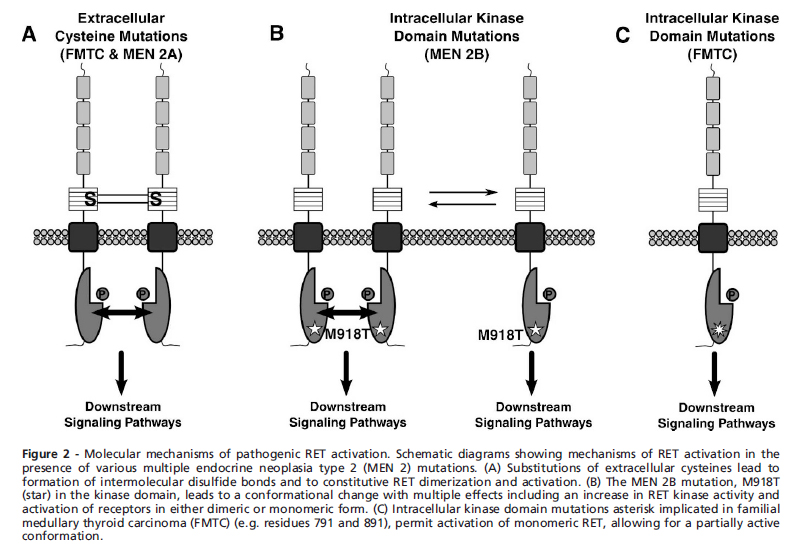
* Germline | [[File:RET kinase domain.jpg|thumb|center|500px|RET kinase domain - [http://www.scielo.br/scielo.php?script=sci_abstract&pid=S1807-59322012001300014&lng=en&nrm=iso&tlng=en Source: Molecular mechanisms of RET receptor-mediated oncogenesis in multiple endocrine neoplasia 2 (Clinics)]]] | ||
* | * Germline [[mutation]]s are responsible for sporadic multiple endocrine neoplasia type 2, while [[mutation]]s in the [[cysteine]] residues in the [[exon]]s of the [[RET gene|RET]] protein product are common in familial multiple endocrine neoplasia type 2.<ref name="WellsPacini20133">{{cite journal|last1=Wells|first1=Samuel A.|last2=Pacini|first2=Furio|last3=Robinson|first3=Bruce G.|last4=Santoro|first4=Massimo|title=Multiple Endocrine Neoplasia Type 2 and Familial Medullary Thyroid Carcinoma: An Update|journal=The Journal of Clinical Endocrinology & Metabolism|volume=98|issue=8|year=2013|pages=3149–3164|issn=0021-972X|doi=10.1210/jc.2013-1204}}</ref> | ||
* The majority of multiple endocrine neoplasia type 2 [[Patient|patients]], derive from a variation in the [[RET proto-oncogene|''RET'' proto-oncogene]], and are specific for cells of [[neural crest]] origin.<ref name="MartuccielloLerone2012">{{cite journal|last1=Martucciello|first1=Giuseppe|last2=Lerone|first2=Margherita|last3=Bricco|first3=Lara|last4=Tonini|first4=Gian|last5=Lombardi|first5=Laura|last6=Del Rossi|first6=Carmine G|last7=Bernasconi|first7=Sergio|title=Multiple endocrine neoplasias type 2B and RET proto-oncogene|journal=Italian Journal of Pediatrics|volume=38|issue=1|year=2012|pages=9|issn=1824-7288|doi=10.1186/1824-7288-38-9}}</ref> | |||
* The RET protooncogene is a 21-exon gene and encodes for a tyrosine kinase transmembrane receptor located on chromosome 10q11.2.<ref | * The [[RET proto-oncogene|''RET'' protooncogene]] is a 21-[[exon]] [[gene]] and encodes for a [[tyrosine kinase]] transmembrane receptor located on [[chromosome]] 10q11.2.<ref>C. Romei, E. Pardi, F. Cetani, and R. Elisei, “Genetic and Clinical Features of Multiple Endocrine Neoplasia Types 1 and 2,” Journal of Oncology, vol. 2012, Article ID 705036, 15 pages, 2012. doi:10.1155/2012/705036</ref> | ||
* Four different ligands have so far been recognized: | * Four different ligands have so far been recognized: The [[glial cell line-derived neutrophilic factor]] (GDNF), [[neurturin]] (NTN), persepin (PNS) and [[artemin]] (ART). The interaction is mediated by a ligand-specific coreceptor (e.g., the GFRα-1 is the co-[[receptor]] for the GDNF).<ref name="SchmutzlerRoy2009">{{cite journal|last1=Schmutzler|first1=B.S.|last2=Roy|first2=S.|last3=Hingtgen|first3=C.M.|title=Glial cell line–derived neurotrophic factor family ligands enhance capsaicin-stimulated release of calcitonin gene-related peptide from sensory neurons|journal=Neuroscience|volume=161|issue=1|year=2009|pages=148–156|issn=03064522|doi=10.1016/j.neuroscience.2009.03.006}}</ref> | ||
* The receptor is composed of an extracellular domain (EC), with a distal cadherin-like region and a juxtamembrane | * The receptor is composed of an extracellular domain (EC), with a distal [[cadherin]]-like region and a juxtamembrane [[cysteine]]-rich region, a transmembrane domain (TM) and an [[intracellular]] domain with tyrosine-kinase activity (TK).<ref name="De FalcoCarlomagno2017">{{cite journal|last1=De Falco|first1=Valentina|last2=Carlomagno|first2=Francesca|last3=Li|first3=Hong-yu|last4=Santoro|first4=Massimo|title=The molecular basis for RET tyrosine-kinase inhibitors in thyroid cancer|journal=Best Practice & Research Clinical Endocrinology & Metabolism|volume=31|issue=3|year=2017|pages=307–318|issn=1521690X|doi=10.1016/j.beem.2017.04.013}}</ref> | ||
< | The table below summarizes specific ''RET'' codons and their functions.<ref name="pmid22584710">{{cite journal| author=Wagner SM, Zhu S, Nicolescu AC, Mulligan LM| title=Molecular mechanisms of RET receptor-mediated oncogenesis in multiple endocrine neoplasia 2. | journal=Clinics (Sao Paulo) | year= 2012 | volume= 67 Suppl 1 | issue= | pages= 77-84 | pmid=22584710 | doi= | pmc=PMC3328826 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22584710 }} </ref><ref name="WellsPacini20132">{{cite journal|last1=Wells|first1=Samuel A.|last2=Pacini|first2=Furio|last3=Robinson|first3=Bruce G.|last4=Santoro|first4=Massimo|title=Multiple Endocrine Neoplasia Type 2 and Familial Medullary Thyroid Carcinoma: An Update|journal=The Journal of Clinical Endocrinology & Metabolism|volume=98|issue=8|year=2013|pages=3149–3164|issn=0021-972X|doi=10.1210/jc.2013-1204}}</ref> | ||
{| style="border: 0px; font-size: 90%; margin: 3px;" align=center | |||
{| style="border: 0px; font-size: 90%; margin: 3px;" align="center" | |||
|+'''''Molecular effects of RET mutations in multiple endocrine neoplasia type2''''' | |+'''''Molecular effects of RET mutations in multiple endocrine neoplasia type2''''' | ||
! style="background: #4479BA; width: 120px;" | {{fontcolor|#FFF|Mutation location}} | ! style="background: #4479BA; width: 120px;" | {{fontcolor|#FFF|Mutation location}} | ||
| Line 35: | Line 61: | ||
! style="background: #4479BA; width: 550px;" | {{fontcolor|#FFF|Phenotype}} | ! style="background: #4479BA; width: 550px;" | {{fontcolor|#FFF|Phenotype}} | ||
|- | |- | ||
! style="background: #DCDCDC;" | ! rowspan="2" style="background: #DCDCDC;" |Extracellular [[cysteine]] rich location | ||
! style="background: #F5F5F5;" | | ! style="background: #F5F5F5;" | | ||
:c609 | :c609 | ||
| Line 44: | Line 70: | ||
! style="background: #F5F5F5;" |Helps to form teritiary structure with the help of disulfide bonds | ! style="background: #F5F5F5;" |Helps to form teritiary structure with the help of disulfide bonds | ||
! style="background: #F5F5F5;" |Alteration in protein folding and maturation | ! style="background: #F5F5F5;" |Alteration in protein folding and maturation | ||
! style="background: #F5F5F5;" | | ! style="background: #F5F5F5;" |Multiple endocrine neoplasia type 2A and familial [[medullary thyroid carcinoma]] (FMTC) | ||
|- | |- | ||
! style="background: #F5F5F5;" |c634 | ! style="background: #F5F5F5;" |c634 | ||
! style="background: #F5F5F5;" |Formation of intramolecular disulfide bonds | ! style="background: #F5F5F5;" |Formation of intramolecular disulfide bonds | ||
! style="background: #F5F5F5;" |Ligand independant dimerization of receptor molecules | ! style="background: #F5F5F5;" |[[Ligand]] independant dimerization of [[receptor]] molecules | ||
! style="background: #F5F5F5;" | | ! style="background: #F5F5F5;" |Multiple endocrine neoplasia type 2A | ||
|- | |- | ||
! style="background: #DCDCDC;" | ! rowspan="6" style="background: #DCDCDC;" |Intracellular [[tyrosine kinase]] domain | ||
! style="background: #F5F5F5;" | | ! style="background: #F5F5F5;" | | ||
:L790 | :L790 | ||
:Y791 | :Y791 | ||
! style="background: #F5F5F5;" |Terminal lobe of RET kinase | ! style="background: #F5F5F5;" |Terminal lobe of ''[[RET proto-oncogene|RET]]'' kinase | ||
! style="background: #F5F5F5;" |Affects ATP binding and interlobe flexibility | ! style="background: #F5F5F5;" |Affects [[ATP]] binding and interlobe flexibility | ||
! style="background: #F5F5F5;" | | ! style="background: #F5F5F5;" |Multiple endocrine neoplasia type 2A and familial medullary thyroid carcinoma (FMTC) | ||
|- | |- | ||
! style="background: #F5F5F5;" | | ! style="background: #F5F5F5;" | | ||
:E768 | :E768 | ||
! style="background: #F5F5F5;" |Close proximity with ATP binding site | ! style="background: #F5F5F5;" |Close proximity with [[ATP]] binding site | ||
! style="background: #F5F5F5;" |Alters interactions within the region | ! style="background: #F5F5F5;" |Alters interactions within the region | ||
! style="background: #F5F5F5;" |FMTC | ! style="background: #F5F5F5;" |Familial [[medullary thyroid carcinoma]] (FMTC) | ||
|- | |- | ||
! style="background: #F5F5F5;" | | ! style="background: #F5F5F5;" | | ||
:V804 | :V804 | ||
! style="background: #F5F5F5;" |Gatekeeper residue that regulates access to ATP binding site | ! style="background: #F5F5F5;" |Gatekeeper residue that regulates access to [[ATP]] binding site | ||
! style="background: #F5F5F5;" |Alters interactions within the region | ! style="background: #F5F5F5;" |Alters interactions within the region | ||
! style="background: #F5F5F5;" |FMTC | ! style="background: #F5F5F5;" |Familial [[medullary thyroid carcinoma]] (FMTC) | ||
|- | |- | ||
! style="background: #F5F5F5;" | | ! style="background: #F5F5F5;" | | ||
:S891 | :S891 | ||
! style="background: #F5F5F5;" |C terminal lobe of kinase | ! style="background: #F5F5F5;" |C terminal lobe of [[kinase]] | ||
! style="background: #F5F5F5;" |Alters activation of loop conformation | ! style="background: #F5F5F5;" |Alters activation of loop conformation | ||
! style="background: #F5F5F5;" | | ! style="background: #F5F5F5;" |Multiple endocrine neoplasia type 2A and familial [[medullary thyroid carcinoma]] (FMTC) | ||
|- | |- | ||
! style="background: #F5F5F5;" | | ! style="background: #F5F5F5;" | | ||
| Line 81: | Line 107: | ||
! style="background: #F5F5F5;" |Situated next to activated loop | ! style="background: #F5F5F5;" |Situated next to activated loop | ||
! style="background: #F5F5F5;" |Local conformational change | ! style="background: #F5F5F5;" |Local conformational change | ||
! style="background: #F5F5F5;" | | ! style="background: #F5F5F5;" |Multiple endocrine neoplasia type 2B | ||
|- | |- | ||
! style="background: #F5F5F5;" | | ! style="background: #F5F5F5;" | | ||
:M918 | :M918 | ||
! style="background: #F5F5F5;" |Substrate binding pocket of the kinase | ! style="background: #F5F5F5;" |Substrate binding pocket of the kinase | ||
! style="background: #F5F5F5;" |Alters protein conformation | ! style="background: #F5F5F5;" |Alters [[protein]] conformation | ||
! style="background: #F5F5F5;" | | ! style="background: #F5F5F5;" |Multiple endocrine neoplasia type 2B | ||
|} | |} | ||
* | * Multiple endocrine neoplasia type 2 generally results from a gain-of-function variant of a ''[[RET proto-oncogene|RET]]'' gene. Other [[disease]]s, such as [[Hirschsprung disease]], result from loss-of-function variants.<ref name="SchmutzlerRoy20092">{{cite journal|last1=Schmutzler|first1=B.S.|last2=Roy|first2=S.|last3=Hingtgen|first3=C.M.|title=Glial cell line–derived neurotrophic factor family ligands enhance capsaicin-stimulated release of calcitonin gene-related peptide from sensory neurons|journal=Neuroscience|volume=161|issue=1|year=2009|pages=148–156|issn=03064522|doi=10.1016/j.neuroscience.2009.03.006}}</ref> | ||
* | *Multiple endocrine neoplasia type 2 is transmitted in an [[autosomal dominant]]. Nevertheless, it can result from spontaneous new mutations in the [[RET gene|''RET'' gene]] with no family history of the disorder, as reported in some cases. For instance, among multiple endocrine neoplasia type 2B, spontaneous new [[mutation]]s were observed in about 50% of the total number of cases. | ||
* Activating germline point | * Activating germline [[point mutation]]s of the ''RET'' [[proto-oncogene]] are causative events in multiple endocrine neoplasia type 2A, multiple endocrine neoplasia type 2B, and familial [[medullary thyroid carcinoma]] (FMTC). ''[[RET]]'' mutations have been found to be widely distributed not only among the 5 [[cysteine]] codons 609, 611, 618, 620, and 634 but also in other noncysteine codons, such as codon 804 in exon 14, codon 883 in exon 15, and others.<ref name="WellsPacini20134">{{cite journal|last1=Wells|first1=Samuel A.|last2=Pacini|first2=Furio|last3=Robinson|first3=Bruce G.|last4=Santoro|first4=Massimo|title=Multiple Endocrine Neoplasia Type 2 and Familial Medullary Thyroid Carcinoma: An Update|journal=The Journal of Clinical Endocrinology & Metabolism|volume=98|issue=8|year=2013|pages=3149–3164|issn=0021-972X|doi=10.1210/jc.2013-1204}}</ref> | ||
* The following | * The following figure depicts the structure and mutation of RET receptor.<ref name="pmid22584710">{{cite journal| author=Wagner SM, Zhu S, Nicolescu AC, Mulligan LM| title=Molecular mechanisms of RET receptor-mediated oncogenesis in multiple endocrine neoplasia 2. | journal=Clinics (Sao Paulo) | year= 2012 | volume= 67 Suppl 1 | issue= | pages= 77-84 | pmid=22584710 | doi= | pmc=PMC3328826 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22584710 }} </ref> | ||
[[File:Genetics of MEN 2.png|thumb|center|500px|Structure, activation and oncogenic mutation of RET receptor. Figure A depicts location of oncogenic mutations of RET recpetor. RET protein has cystine rich extracellular domain, cadherin homology domain, transmembrane domain and an intracellular tyrosine kinase domain. FIgure B depicts RET activation]] | [[File:Genetics of MEN 2.png|thumb|center|500px|Structure, activation and oncogenic mutation of RET receptor. Figure A depicts location of oncogenic mutations of RET recpetor. RET protein has cystine rich extracellular domain, cadherin homology domain, transmembrane domain and an intracellular tyrosine kinase domain. FIgure B depicts RET activation - [http://www.scielo.br/scielo.php?script=sci_abstract&pid=S1807-59322012001300014&lng=en&nrm=iso&tlng=en Source: Molecular mechanisms of RET receptor-mediated oncogenesis in multiple endocrine neoplasia 2 (Clinics)]]] | ||
===RET Activation=== | ===RET Activation=== | ||
* Dimerization of RET | * Dimerization of [[RET proto-oncogene|''RET'' proto-oncogene]] mediated through formation of multicomponent complex. [[RET proto-oncogene|''RET'' proto-oncogene]] is activated by binding both a soluble ligand and a non signaling [[extracellular]] co-receptor. ''[[RET gene|RET]]'' activation leads to phosphorylation of multiple [[intracellular]] [[tyrosine kinase]]. | ||
[[File:Mutations specificities.jpg|thumb|center|500px|MEN type 2 mutations - [http://www.scielo.br/scielo.php?script=sci_abstract&pid=S1807-59322012001300014&lng=en&nrm=iso&tlng=en Source: Molecular mechanisms of RET receptor-mediated oncogenesis in multiple endocrine neoplasia 2 (Clinics)]]] | |||
==Associated Conditions== | ==Associated Conditions== | ||
Some of the | * Some of the [[disease]]s specific to the [[gene]]s of multiple endocrine neoplasia type 2 are as follows.<ref name="pmid22584710">{{cite journal| author=Wagner SM, Zhu S, Nicolescu AC, Mulligan LM| title=Molecular mechanisms of RET receptor-mediated oncogenesis in multiple endocrine neoplasia 2. | journal=Clinics (Sao Paulo) | year= 2012 | volume= 67 Suppl 1 | issue= | pages= 77-84 | pmid=22584710 | doi= | pmc=PMC3328826 | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=22584710 }} </ref> | ||
{| style="border: 0px; font-size: 90%; margin: 3px;" align=center | {| style="border: 0px; font-size: 90%; margin: 3px;" align="center" | ||
|+'''''Associated tumors''''' | |+'''''Associated tumors''''' | ||
! style="background: #4479BA; width: 120px;" | {{fontcolor|#FFF|Subtype}} | ! style="background: #4479BA; width: 120px;" | {{fontcolor|#FFF|Subtype}} | ||
! style="background: #4479BA; width: 550px;" | {{fontcolor|#FFF|Associated diseases}} | ! style="background: #4479BA; width: 550px;" | {{fontcolor|#FFF|Associated diseases}} | ||
|- | |- | ||
! style="background: #F5F5F5;" | | ! style="background: #F5F5F5;" |Multiple endocrine neoplasia type 2A | ||
! style="background: #F5F5F5;" |Cutaneous lichen amyloidosis, Hirschsprung disease | ! style="background: #F5F5F5;" |Cutaneous lichen [[amyloidosis]], [[Hirschsprung disease]] | ||
|- | |- | ||
! style="background: #F5F5F5;" | | ! style="background: #F5F5F5;" |Multiple endocrine neoplasia type 2B | ||
! style="background: #F5F5F5;" |Ganglioneuromatosis, marfanoid habitus | ! style="background: #F5F5F5;" |Ganglioneuromatosis, marfanoid habitus | ||
|- | |- | ||
! style="background: #F5F5F5;" |FMTC | ! style="background: #F5F5F5;" |Familial medullary thyroid carcinoma (FMTC) | ||
! style="background: #F5F5F5;" |Rare diseases | ! style="background: #F5F5F5;" |Rare diseases | ||
|} | |} | ||
==Gross Pathology== | ===Gross Pathology=== | ||
<gallery> | <gallery> | ||
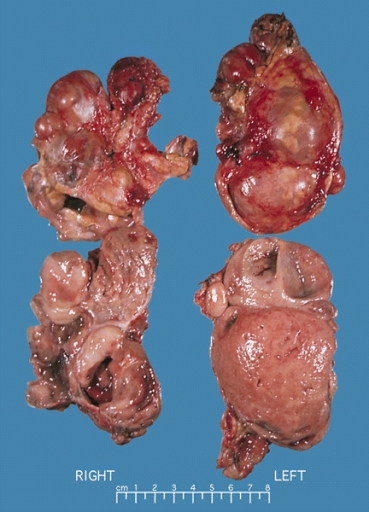
Image:Bilateral pheo MEN2.jpg|ADRENAL GLAND: BILATERAL PHEOCHROMOCYTOMA Cross section of bilateral pheochromocytomas from a 30-year-old man with MEN syndrome type IIa. The right adrenal tumor weighed 168 g and the left 220 g. Note the distinct multinodular, multicentric pattern of growth on both sides | Image:Bilateral pheo MEN2.jpg|ADRENAL GLAND: BILATERAL PHEOCHROMOCYTOMA Cross section of bilateral pheochromocytomas from a 30-year-old man with MEN syndrome type IIa. The right adrenal tumor weighed 168 g and the left 220 g. Note the distinct multinodular, multicentric pattern of growth on both sides [https://upload.wikimedia.org/wikipedia/commons/5/5f/Bilateral_pheo_MEN2.jpg Source: Wikimedia Commons] | ||
Image:Pheochromocytoma 03.jpeg|Pheochromocytoma, | Image:Pheochromocytoma 03.jpeg|Pheochromocytoma, Image courtesy of Dr Frank Gaillard<ref name=radio02>Image courtesy of Dr Frank Gaillard. [http://www.radiopaedia.org Radiopaedia] (original file[http://radiopaedia.org/cases/10816‘’here’’]).[http://radiopaedia.org/licence Creative Commons BY-SA-NC]</ref> | ||
Image:Pheochromocytoma 04.JPG|Pheochromocytoma, | Image:Pheochromocytoma 04.JPG|Pheochromocytoma, Image courtesy of Dr Frank Gaillard<ref name=radio02>Image courtesy of Dr Frank Gaillard. [http://www.radiopaedia.org Radiopaedia] (original file[http://radiopaedia.org/cases/10816‘’here’’]).[http://radiopaedia.org/licence Creative Commons BY-SA-NC]</ref> | ||
</gallery> | </gallery> | ||
==Microscopic Pathology== | ===Microscopic Pathology=== | ||
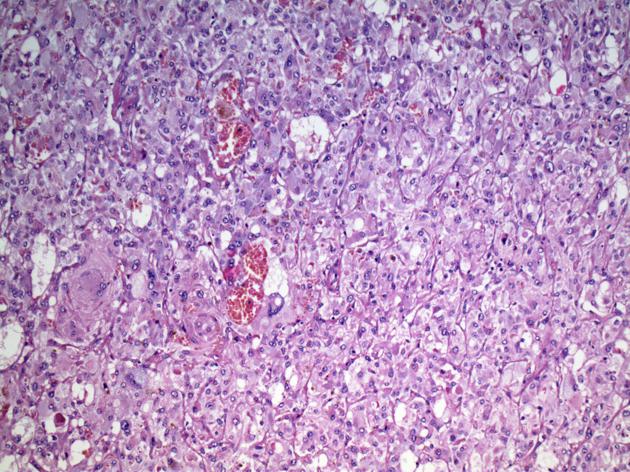
===Medullary Carcinoma of Thyroid=== | ====Medullary Carcinoma of Thyroid==== | ||
* Nests of C | * Nests of [[C cell]]s invading the [[basement membrane]] and infiltrating [[thyroid]] follicles | ||
<gallery> | <gallery> | ||
Image:Pheochromocytoma histology.jpg|Pheochromocytoma, Case courtesy of Dr Andrew Ryan, [http://radiopaedia.org/ Radiopaedia.org From the case <a href="http://radiopaedia.org/cases/22683">rID: 22683] | |||
Image:Pheochromocytoma histology.jpg|Pheochromocytoma, Case courtesy of Dr Andrew Ryan, | |||
</gallery> | </gallery> | ||
===Histopathological Video=== | |||
====Video==== | |||
{{#ev:youtube|P2uPUbDPbuI}} | |||
{{#ev:youtube|7yjxG3KmX98}} | |||
{{#ev:youtube|crwGfnWKEZ8}} | |||
==References== | ==References== | ||
{{reflist|2}} | {{reflist|2}} | ||
[[Category:Endocrinology]] | [[Category:Endocrinology]] | ||
[[Category:Oncology]] | |||
[[CAtegory:Diseases]] | |||
[[Category:Medicine]] | |||
[[Category:Endocrinology]] | |||
[[Category:Up-To-Date]] | |||
{{WikiDoc Help Menu}} | {{WikiDoc Help Menu}} | ||
{{WikiDoc Sources}} | {{WikiDoc Sources}} | ||
[[Category:Up-To-Date]] | |||
[[Category:Oncology]] | |||
[[Category:Medicine]] | |||
[[Category:Endocrinology]] | |||
[[Category:Surgery]] | |||
Latest revision as of 20:11, 6 July 2019
|
Multiple endocrine neoplasia type 2 Microchapters |
|
Differentiating Multiple endocrine neoplasia type 2 from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Multiple endocrine neoplasia type 2 pathophysiology On the Web |
|
American Roentgen Ray Society Images of Multiple endocrine neoplasia type 2 pathophysiology |
|
Multiple endocrine neoplasia type 2 pathophysiology in the news |
|
Blogs on Multiple endocrine neoplasia type 2 pathophysiology |
|
Directions to Hospitals Treating Multiple endocrine neoplasia type 2 |
|
Risk calculators and risk factors for Multiple endocrine neoplasia type 2 pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [3]
Overview
The progression to multiple endocrine neoplasia type 2 usually involves the genetic mutations. The pathogenesis of multiple endocrine neoplasia type 2 involves a mutation of the RET gene.
Pathogenesis
The common feature among the three subtypes of multiple endocrine neoplasia type 2 is a high propensity to develop medullary thyroid carcinoma.
Multiple Endocrine Neoplasia type 2A
- Multiple endocrine neoplasia type 2 (MEN2) generally occur in endocrine organs (e.g. thyroid, parathyroid, and adrenals), but may also occur in endocrine tissues of organs not classically thought of as endocrine.[1]
- Although many different types of hormone-producing tumors are associated with multiple endocrine neoplasia type 2, the most common manifestation is a form of thyroid cancer called medullary thyroid carcinoma. This tumor secretes an inactive hormone called calcitonin. [2]
- Many people with this disorder also develop pheochromocytoma, which is a tumor of the adrenal glands (located above each kidney) that can cause dangerously high blood pressure. In addition, overactivity of the parathyroid gland (hyperparathyroidism) occurs in some cases of multiple endocrine neoplasia type 2. Hyperparathyroidism disrupts the normal balance of calcium in the blood, which can lead to kidney stones, thinning of bones, weakness, and fatigue.[3]
- In multiple endocrine neoplasia type 2, primary hyperparathyroidism occurs in only 10–30% and is usually diagnosed after the third decade of life. It can occur in children but this is rare. It may be the sole clinical manifestation of this syndrome but this is unusual.[4]
- Multiple endocrine neoplasia type 2 associates medullary thyroid carcinoma with pheochromocytoma in about 20–50% of cases and with primary hyperparathyroidism in 5–20% of cases.[5]
- Other components of the disease are absent in familial isolated medullary thyroid carcinoma.[6]
Multiple Endocrine Neoplasia type 2B
- Multiple endocrine neoplasia type 2B is associated with medullary thyroid carcinoma and pheochromocytoma as well as with marfanoid habitus and with mucosal and digestive neurofibromatosis.
| Subtype | Medullary Thyroid Carcinoma | Pheochromocytoma | Parathyroid Disease |
|---|---|---|---|
| Multiple endocrine neoplasia type 2A | 95% | 50% | 20% to 30% |
| Multiple endocrine neoplasia type 2B | 100% | 50% | Uncommon |
| Familial medullary thyroid carcinoma | 100% | 0% | 0% |
Genetics
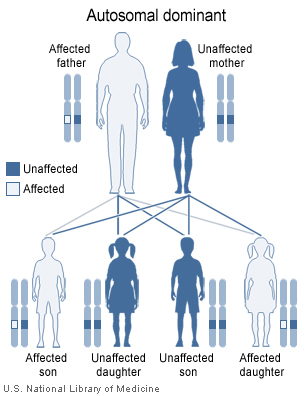
- Most cases of multiple endocrine neoplasia type 2 are inherited in an autosomal dominant pattern, which means affected people may have affected siblings and relatives in successive generations (such as parents and children). An affected person usually has one parent with the condition. Some cases, however, result from new mutations in the RET proto-oncogene. These cases occur in people with no history of the disorder in their family.

- Germline mutations are responsible for sporadic multiple endocrine neoplasia type 2, while mutations in the cysteine residues in the exons of the RET protein product are common in familial multiple endocrine neoplasia type 2.[7]
- The majority of multiple endocrine neoplasia type 2 patients, derive from a variation in the RET proto-oncogene, and are specific for cells of neural crest origin.[8]
- The RET protooncogene is a 21-exon gene and encodes for a tyrosine kinase transmembrane receptor located on chromosome 10q11.2.[9]
- Four different ligands have so far been recognized: The glial cell line-derived neutrophilic factor (GDNF), neurturin (NTN), persepin (PNS) and artemin (ART). The interaction is mediated by a ligand-specific coreceptor (e.g., the GFRα-1 is the co-receptor for the GDNF).[10]
- The receptor is composed of an extracellular domain (EC), with a distal cadherin-like region and a juxtamembrane cysteine-rich region, a transmembrane domain (TM) and an intracellular domain with tyrosine-kinase activity (TK).[11]
The table below summarizes specific RET codons and their functions.[12][13]
| Mutation location | RET codons | Function of wild type codon | Mutated effects | Phenotype |
|---|---|---|---|---|
| Extracellular cysteine rich location |
|
Helps to form teritiary structure with the help of disulfide bonds | Alteration in protein folding and maturation | Multiple endocrine neoplasia type 2A and familial medullary thyroid carcinoma (FMTC) |
| c634 | Formation of intramolecular disulfide bonds | Ligand independant dimerization of receptor molecules | Multiple endocrine neoplasia type 2A | |
| Intracellular tyrosine kinase domain |
|
Terminal lobe of RET kinase | Affects ATP binding and interlobe flexibility | Multiple endocrine neoplasia type 2A and familial medullary thyroid carcinoma (FMTC) |
|
Close proximity with ATP binding site | Alters interactions within the region | Familial medullary thyroid carcinoma (FMTC) | |
|
Gatekeeper residue that regulates access to ATP binding site | Alters interactions within the region | Familial medullary thyroid carcinoma (FMTC) | |
|
C terminal lobe of kinase | Alters activation of loop conformation | Multiple endocrine neoplasia type 2A and familial medullary thyroid carcinoma (FMTC) | |
|
Situated next to activated loop | Local conformational change | Multiple endocrine neoplasia type 2B | |
|
Substrate binding pocket of the kinase | Alters protein conformation | Multiple endocrine neoplasia type 2B |
- Multiple endocrine neoplasia type 2 generally results from a gain-of-function variant of a RET gene. Other diseases, such as Hirschsprung disease, result from loss-of-function variants.[14]
- Multiple endocrine neoplasia type 2 is transmitted in an autosomal dominant. Nevertheless, it can result from spontaneous new mutations in the RET gene with no family history of the disorder, as reported in some cases. For instance, among multiple endocrine neoplasia type 2B, spontaneous new mutations were observed in about 50% of the total number of cases.
- Activating germline point mutations of the RET proto-oncogene are causative events in multiple endocrine neoplasia type 2A, multiple endocrine neoplasia type 2B, and familial medullary thyroid carcinoma (FMTC). RET mutations have been found to be widely distributed not only among the 5 cysteine codons 609, 611, 618, 620, and 634 but also in other noncysteine codons, such as codon 804 in exon 14, codon 883 in exon 15, and others.[15]
- The following figure depicts the structure and mutation of RET receptor.[12]

RET Activation
- Dimerization of RET proto-oncogene mediated through formation of multicomponent complex. RET proto-oncogene is activated by binding both a soluble ligand and a non signaling extracellular co-receptor. RET activation leads to phosphorylation of multiple intracellular tyrosine kinase.
Associated Conditions
- Some of the diseases specific to the genes of multiple endocrine neoplasia type 2 are as follows.[12]
| Subtype | Associated diseases |
|---|---|
| Multiple endocrine neoplasia type 2A | Cutaneous lichen amyloidosis, Hirschsprung disease |
| Multiple endocrine neoplasia type 2B | Ganglioneuromatosis, marfanoid habitus |
| Familial medullary thyroid carcinoma (FMTC) | Rare diseases |
Gross Pathology
-
ADRENAL GLAND: BILATERAL PHEOCHROMOCYTOMA Cross section of bilateral pheochromocytomas from a 30-year-old man with MEN syndrome type IIa. The right adrenal tumor weighed 168 g and the left 220 g. Note the distinct multinodular, multicentric pattern of growth on both sides Source: Wikimedia Commons
-
Pheochromocytoma, Image courtesy of Dr Frank Gaillard[16]
-
Pheochromocytoma, Image courtesy of Dr Frank Gaillard[16]
Microscopic Pathology
Medullary Carcinoma of Thyroid
- Nests of C cells invading the basement membrane and infiltrating thyroid follicles
-
Pheochromocytoma, Case courtesy of Dr Andrew Ryan, Radiopaedia.org From the case <a href="http://radiopaedia.org/cases/22683">rID: 22683
Histopathological Video
Video
{{#ev:youtube|P2uPUbDPbuI}}
{{#ev:youtube|7yjxG3KmX98}}
{{#ev:youtube|crwGfnWKEZ8}}
References
- ↑ Moline J, Eng C. (2011). "Multiple endocrine neoplasia type 2: an overview". Genet Med. 9 (13): 755–64. doi:10.1097/GIM.0b013e318216cc6d. PMID 21552134.
- ↑ Cote, Gilbert J.; Grubbs, Elizabeth G.; Hofmann, Marie-Claude (2015). "Thyroid C-Cell Biology and Oncogenic Transformation". 204: 1–39. doi:10.1007/978-3-319-22542-5_1. ISSN 0080-0015.
- ↑ Kantorovich, Vitaly; Pacak, Karel (2010). "Pheochromocytoma and Paraganglioma". 182: 343–373. doi:10.1016/S0079-6123(10)82015-1. ISSN 0079-6123.
- ↑ Wells, Samuel A.; Pacini, Furio; Robinson, Bruce G.; Santoro, Massimo (2013). "Multiple Endocrine Neoplasia Type 2 and Familial Medullary Thyroid Carcinoma: An Update". The Journal of Clinical Endocrinology & Metabolism. 98 (8): 3149–3164. doi:10.1210/jc.2013-1204. ISSN 0021-972X.
- ↑ Almeida, Madson Q.; Stratakis, Constantine A. (2010). "Solid tumors associated with multiple endocrine neoplasias". Cancer Genetics and Cytogenetics. 203 (1): 30–36. doi:10.1016/j.cancergencyto.2010.09.006. ISSN 0165-4608.
- ↑ Niccoli-Sire, P.; Conte-Devolx, B. (2007). "Néoplasies endocriniennes multiples de type 2". Annales d'Endocrinologie. 68 (5): 317–324. doi:10.1016/j.ando.2007.04.005. ISSN 0003-4266.
- ↑ Wells, Samuel A.; Pacini, Furio; Robinson, Bruce G.; Santoro, Massimo (2013). "Multiple Endocrine Neoplasia Type 2 and Familial Medullary Thyroid Carcinoma: An Update". The Journal of Clinical Endocrinology & Metabolism. 98 (8): 3149–3164. doi:10.1210/jc.2013-1204. ISSN 0021-972X.
- ↑ Martucciello, Giuseppe; Lerone, Margherita; Bricco, Lara; Tonini, Gian; Lombardi, Laura; Del Rossi, Carmine G; Bernasconi, Sergio (2012). "Multiple endocrine neoplasias type 2B and RET proto-oncogene". Italian Journal of Pediatrics. 38 (1): 9. doi:10.1186/1824-7288-38-9. ISSN 1824-7288.
- ↑ C. Romei, E. Pardi, F. Cetani, and R. Elisei, “Genetic and Clinical Features of Multiple Endocrine Neoplasia Types 1 and 2,” Journal of Oncology, vol. 2012, Article ID 705036, 15 pages, 2012. doi:10.1155/2012/705036
- ↑ Schmutzler, B.S.; Roy, S.; Hingtgen, C.M. (2009). "Glial cell line–derived neurotrophic factor family ligands enhance capsaicin-stimulated release of calcitonin gene-related peptide from sensory neurons". Neuroscience. 161 (1): 148–156. doi:10.1016/j.neuroscience.2009.03.006. ISSN 0306-4522.
- ↑ De Falco, Valentina; Carlomagno, Francesca; Li, Hong-yu; Santoro, Massimo (2017). "The molecular basis for RET tyrosine-kinase inhibitors in thyroid cancer". Best Practice & Research Clinical Endocrinology & Metabolism. 31 (3): 307–318. doi:10.1016/j.beem.2017.04.013. ISSN 1521-690X.
- ↑ 12.0 12.1 12.2 Wagner SM, Zhu S, Nicolescu AC, Mulligan LM (2012). "Molecular mechanisms of RET receptor-mediated oncogenesis in multiple endocrine neoplasia 2". Clinics (Sao Paulo). 67 Suppl 1: 77–84. PMC 3328826. PMID 22584710.
- ↑ Wells, Samuel A.; Pacini, Furio; Robinson, Bruce G.; Santoro, Massimo (2013). "Multiple Endocrine Neoplasia Type 2 and Familial Medullary Thyroid Carcinoma: An Update". The Journal of Clinical Endocrinology & Metabolism. 98 (8): 3149–3164. doi:10.1210/jc.2013-1204. ISSN 0021-972X.
- ↑ Schmutzler, B.S.; Roy, S.; Hingtgen, C.M. (2009). "Glial cell line–derived neurotrophic factor family ligands enhance capsaicin-stimulated release of calcitonin gene-related peptide from sensory neurons". Neuroscience. 161 (1): 148–156. doi:10.1016/j.neuroscience.2009.03.006. ISSN 0306-4522.
- ↑ Wells, Samuel A.; Pacini, Furio; Robinson, Bruce G.; Santoro, Massimo (2013). "Multiple Endocrine Neoplasia Type 2 and Familial Medullary Thyroid Carcinoma: An Update". The Journal of Clinical Endocrinology & Metabolism. 98 (8): 3149–3164. doi:10.1210/jc.2013-1204. ISSN 0021-972X.
- ↑ 16.0 16.1 Image courtesy of Dr Frank Gaillard. Radiopaedia (original file[1]).Creative Commons BY-SA-NC
![Pheochromocytoma, Image courtesy of Dr Frank Gaillard[16]](/images/3/38/Pheochromocytoma_03.jpeg)
![Pheochromocytoma, Image courtesy of Dr Frank Gaillard[16]](/images/6/6a/Pheochromocytoma_04.JPG)