Low density lipoprotein future or investigational therapies
|
Low Density Lipoprotein Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Low density lipoprotein future or investigational therapies On the Web |
|
American Roentgen Ray Society Images of Low density lipoprotein future or investigational therapies |
|
FDA on Low density lipoprotein future or investigational therapies |
|
CDC on Low density lipoprotein future or investigational therapies |
|
Low density lipoprotein future or investigational therapies in the news |
|
Blogs on Low density lipoprotein future or investigational therapies |
|
Risk calculators and risk factors for Low density lipoprotein future or investigational therapies |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ayokunle Olubaniyi, M.B,B.S [2]
Overview
There is a direct relationship between levels of circulating LDL cholesterol and the risk of cardiovascular disease. There is an unmet need for more effective and tolerable therapies for reducing LDL-C, which is a direct consequence of failures observed with the available therapies at achieving target LDL-C levels. This section describes novel therapeutic targets currently under development, including anti-sense oligonucleotides (ASOs) to apolipoprotein B (apo B), proprotein convertase subtilisin/kexin type 9 (PCSK9), microsomal triglyceride transfer protein (MTP), thyromimetics, squalene synthase, adenosine triphosphate-citrate lyase, AMP-activated protein kinase, and sterol regulatory element binding proteins.
The Unmet Need Driving Research Towards a Lower LDL Cholesterol Goal
Although the relative rate of death attributable to cardiovascular disease declined by 32.7% in the past decade, the burden of disease remains very high at 1 out of every 3 deaths in the United States,[1] and the direct medical cost is expected to triple by 2030.[2] LDL cholesterol remains the dominant determinant of cardiovascular heart diseases,[3] thus making statins (HMG CoA reductase inhibitors) central in the fight against atherosclerosis. However, 40% and 80% of individuals with high- and very-high cardiovascular risk, respectively, still do not achieve their LDL-C goals with optimal doses of statins.[4] Therefore, alternative therapeutic targets to effectively and safely reduce LDL cholesterol are actively being investigated.
Investigational Therapies
Inhibition of Apolipoprotein B Production
Apolipoprotein B (apo B) is a large protein that is present in all atherogenic lipoproteins i.e., VLDL, LDL, IDL. There is a single copy of apo B-100 in all these lipoproteins, therefore, plasma levels of apo B-100 is proportionate to the concentration of circulating atherogenic lipoproteins and a predictor of cardiovascular risk.[5] From the apoB gene, the liver synthesizes apo B-100 and the intestine synthesizes apo B-48 which is required for chylomicron assemly and fat absorption. The apo B-100 serves two functions - provides structural stability to the circulating lipoproteins as well as acts as a ligand for LDL receptors (LDLR). The removal of LDL from the plasma involves the binding of apo B to LDLR, then, the resulting apo B-100-LDLR complex gets internalized into the liver for processing.[6] Mutations that lower the affinity of apo B-100 for LDLR result in decreased clearance of LDLs, a condition known as familial defective apo B with an increased risk of atherosclerotic cardiovascular diseases.[7][8] In contrast, mutations in apo B that decrease its translation or secretion, or increase its breakdown have been demonstrated to reduce the circulating LDL-C and improve cardiovascular risk.[9]
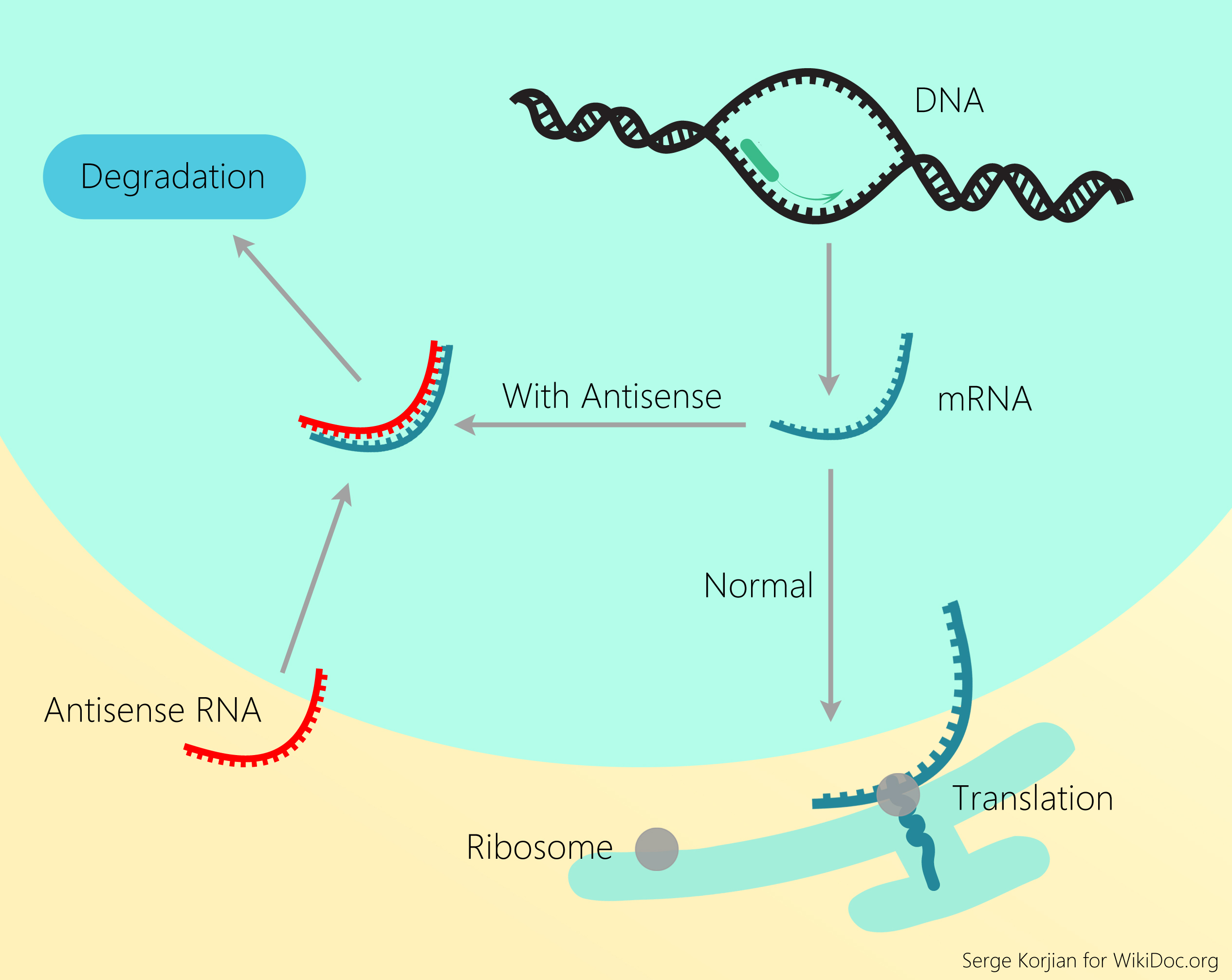
The DNA contains two strands - 'sense' and 'antisense' which run complementary to each other. The antisense strand encodes a sequence of events that initiates protein synthesis and production of messenger RNA (mRNA) which later serves as a template for protein synthesis through a process called translation. Antisense oligoneucleotides (ASOs) are short, deoxyribonucleotide strands which bind to the targeted mRNAs to inhibit gene expression. They can inhibit mRNA translation and mRNA splicing, thus, leading to its enzymatic degradation by ribonuclease (RNAse H or argonaute 2).[10]
ISIS 301012 or mipomersen, by ISIS Pharmaceuticals, is a second-generation 20 nucleotide ASO which selectively inhibits apo B gene expression via RNAse H activation.[11] Phases I and II clinical trials have demonstrated a dose-dependent reduction of plasma apo B levels by 40% and up to 50% reduction in LDL-C of a subcutaneously administered ISIS-301012 or mipomersen, even with a defective LDLR.[12][13][14] Furthermore, a phase III randomized clinical trial involving homozygous FH revealed a 15% elevation in HDL-C.[15] Despite its efficacy in lowering LDL-C, its approval has been hampered with the development of adverse effects - injection site reactions (80-100% of patients), flu-like illness, and 3-fold elevation in liver transaminases (15%).
PCSK9 Inhibition
PCSK9 has a major role in the metabolism of hepatic cholesterol. It is a serine protease which binds to the epidermal growth factor-like repeat A (EGF-A) domain of the low-density lipoprotein receptor (LDLR), inducing LDLR degradation in the lysosomes. Reduced LDL receptor levels result in decreased metabolism of low density lipoprotein (LDL), which could lead to hypercholesterolemia.[16] The sterol regulatory element-binding protein-2 (SREBP-2), which is activated in the presence of low intracellular levels of cholesterol, also induces the expression of PCSK9, thereby increasing the amount of circulating LDL-cholesterol.[17] In addition to the effect on LDL-C, PCSK9 deficiency has also been shown to lower cardiovascular risk factors by reducing postprandial triglyceridemia.[18] In another study, PCSK9-deficient mice were also demonstrated to have a reduced lymphatic apoB secretion,[19] as well as an increased ability to clear chylomicrons.
For more information regarding this novel approach to lipid management, Click here.
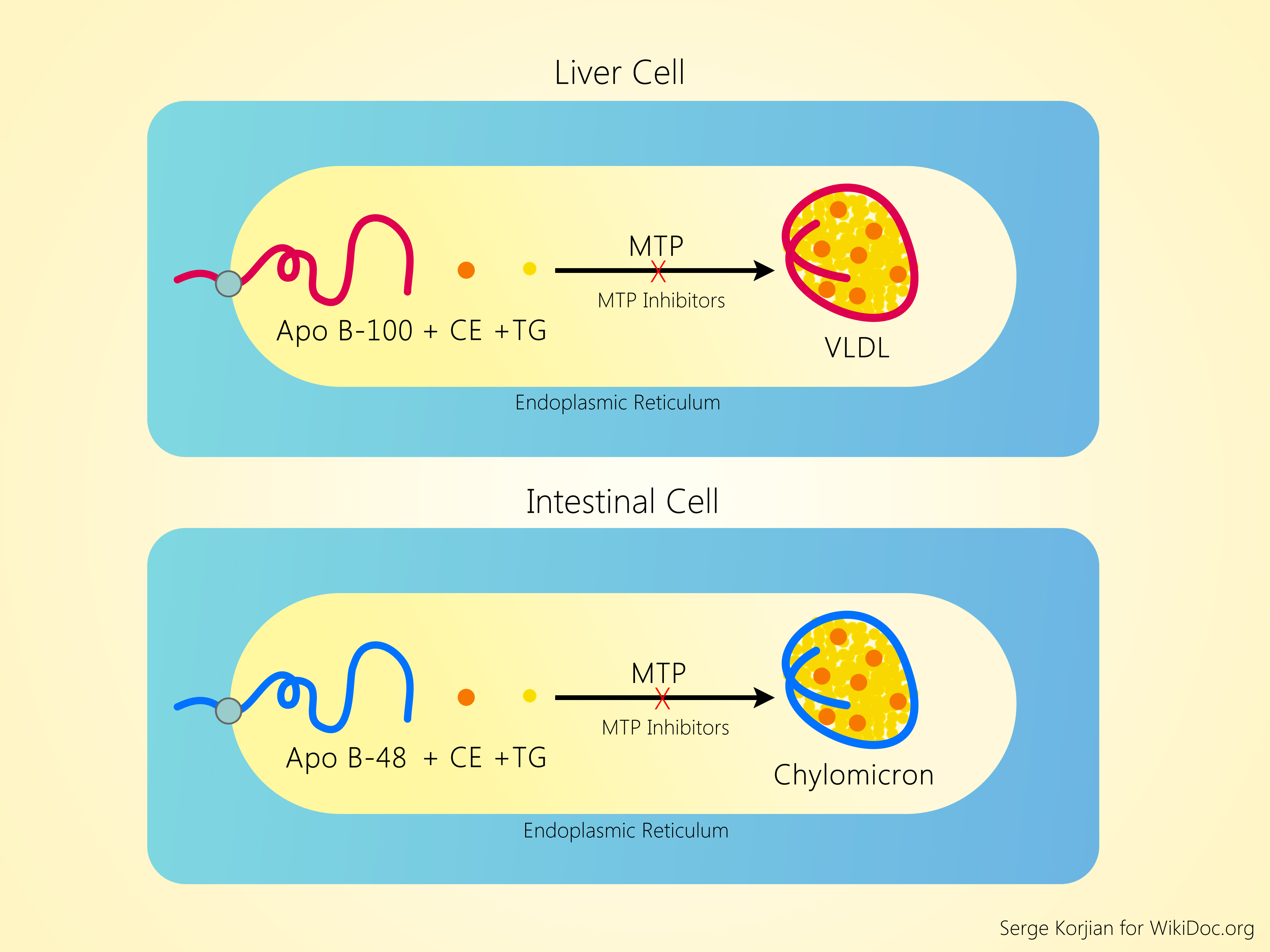
Microsomal Triglyceride Transfer Protein (MTP) Inhibition
Microsomal triglyceride transfer protein is an endosomal protein found in the hepatocytes and intestinal enterocytes. It catalyses the transfer of cholesterol esters and triglycerides to nascent apo B, leading to the formation of chylomicron and VLDL in the intestine and hepatocyte, respectively.[20] Chylomicrons and other apo-B48-containing remnant lipoproteins are essential for intestinal fat absorption and its transfer to peripheral tissues. Mutations of the MTTP gene leads to a condition known as abetalipoproteinemia, which causes an absence of apo-B-containing lipoproteins and very low levels of LDL-C and triglycerides.[21][22] Individuals with this recessive condition have severe intestinal malabsorption of fat and fat-soluble vitamins (A, D, E, K) manifesting as fatty liver, night-blindness, rickets or osteomalacia, neuropathy, ataxia, and coagulopathy.
Many MTP inhibitors are being investigated, they are:
- Non-intestinal specific agentS
- Lomitapide (AEGR-427, previously known as BMS-201038 - Bristol-Myers-Squibb) by Aegerion Pharmaceuticals
- Implitapide (formerly AEGR-427 or Bayer BAY-13-9952)
- CP-34086 by Pfizer
- Intestinal-specific agents
- Dirlotapide by Pfizer
- JTT-130 and SLx-4090 by Surface Logix.
A clinical trial assessing the efficacy of lometapide in 6 patients with homozygous familial hypercholesterolemia demonstrated a 58%, 51%, and 55% reductions in total cholesterol, LDL-C and apo B respectively.[23] Higher doses were associated with a transient elevation of liver transaminases and hepatic fat. A double-blinded, randomized controlled trial involving 84 subjects further demonstrated a 20%, 30%, and 46% reduction in LDL-C in ezetimide alone, lometapide alone, and lometapide plus ezetimide combination study groups respectively, further emphasizing the beneficial effect of lometapide monotherapy or in combination therapy which was associated with significantly greater reductions in LDL-C levels compared with ezetimibe monotherapy.[24] Another MTP inhibitor, CP-34086 by Pfizer, showed a 47% and 72% reduction in total cholesterol and LDL-C in healthy human volunteers respectively.[25] Currently, further developments of CP-34086 and implitapide have been placed on hold due to their hepatic adverse effects.
Intestinal-specific agents such as dirlotapide, JTT-130, and SLx-4090 were developed to prevent the hepatic effects of the non-specific agents. Thus far, they are still early in human clinical trials (except dirlotapide) but there were reports of significant reductions in postprandial triglyceridemia and total cholesterol in preclinical animal studies with dirlotapide.[26] Intestinal-specific MTP inhibitors may be effective in treating hyperchylomicronemia but their efficacy as LDL-C lowering agents is uncertain.
Thyromimetics
The association between thyroid hormones and cholesterol metabolism was first discovered by Mason in the 1930s, and its use as a cholesterol lowering agent has been investigated in several studies.[27][28][29] However, their development have been hindered by the associated adverse cardiac effects, which was mainly due to the contamination of the investigated thyroid hormone, dextrothyroxine (DT4) with levothyroxine (LT4),[30][31] and by the birth of statins. The recent discovery of thyroid hormone receptors (TRs) have brought this approach back into existence. There are two main TRs in humans:
- TRα receptors (TRα 1 & 2). TRα 1 is predominantly in the muscles and adipose tissue; also mediates the cardiovascular responses to thyroid hormones such as tachycardia.[32]
- TRβ receptors (TRβ 1 & 2). TRβ 1 is mainly in the liver and it regulates cholesterol homeostasis.[32] Therefore, the development of TRβ 1-specific thyromimetic would be a promising method of cholesterol management devoid of cardiac effects.[33][34]
Some of the proposed mechanisms of action of these agents include:
- Upregualtion of hepatic LDLR expression by TRβ.[35][36]
- Stimulation of bile acid synthesis through the upregulation of the rate-limiting enzyme, cholesterol 7-hydroxylase [CYP7A1])
- Stimulation of biliary excretion (through increased expression of ATP-binding cassette proteins G5/G8 [ABCG5/G8]
- Promotion of reverse cholesterol transport which ultimately increases the formation of HDL, enhances cholesteryl ester transfer protein (CETP) activity, and increases scavenger receptor B-I (SR-BI) activity for the uptake of cholesterol.
Examples of TRβ 1-specific thyromimetics that had been investigated include:
- DITPA (3,5-diiodothyropropionic acid) - terminated
- Eprotirome (KB2115)[37] by Karo Bio AB.
- Sobetirome (GC-1)
- MB07811
- KB-141
- T-0681
Squalene Synthase Inhibition
Similar to the statins (3-hydroxy-3-methylglutaryl-CoA reductase inhibitors), inhibitors of squalene synthase prevent the conversion of farnesyl pyrophosphate to squalene at a point on the HMG-CoA-Mevalonate pathway which represents the commitment of cholesterol intermediates to the synthesis of cholesterol. Squalene synthase inhibitors have been shown to inhibit cholesterol production, reduce triglyceride synthesis and apoB secretion, increase LDL receptor expression and LDL uptake in HepG2 cells.[38] However, they are less likely to be associated with the adverse myopathic effects commonly observed with statins because they do not cause depletion of isoprenoid intermediates within the cholesterol biosynthesis pathway, and as a result, they do not limit the prenylation or lipidation (addition of hydrophobic molecules to a protein) of membrane-directed proteins.[39]
TAK-475 (lapaquistat acetate) by Takeda Pharmaceuticals was the first squalene synthase inhibitor to reach phase III clinical trials for the treatment of hypercholesterolemia in the United States and Europe. Randomized, double-blinded, placebo and actively controlled, parallel-group studies involving TAK-475 alone and in combination with atovastatin were associated with a dose-dependent reduction of LDL-C up to 27% and 19% when compared with placebo and when combined with atovastatin, respectively in healthy human volunteers. Recent animal studies have demonstrated a protective effect against statin-induced myopathy when isoprenoid intermediates are replenished directly or by the use of TAK-475 given with high-dose statins.[40] Results from these studies further underscore squalene synthase inhibitors as potential drugs to clinically prevent statin-induced myopathies.
Phase III multi-centered clinical trials are on-going to compare TAK-475 vs simvastatin alone or in combination, vs. ezetimibe, and as add-on in patients already on atorvastatin, rosuvastatin, or a low or high-dose statin. It will also be investigated as add-on treatment in patients with homozygous familial hypercholesterolemia, and in patients with type 2 diabetes.
Inhibition of ACL and Activation of AMPK
ETC-1002 (8-hydroxy-2,2,14,14-tetramethylpentadecanedioic acid), by Esperion Therapeutics, is a small molecule which regulates lipid and carbohydrate metabolism. It modulates the activity of two distinct molecular targets - hepatic adenosine triphosphate-citrate lyase (ACL) and AMP-activated protein kinase (AMPK).[41] It works by:
- Inhibition of ACL - Inhibition of adenosine triphosphate-citrate lyase, an enzyme responsible for the production of ATP citrate, reduces the levels of acetyl co-enzyme A (acetyl-CoA - an important precursor to HMG-CoA which is a vital component in cholesterol and ketone synthesis). It acts on the lipid synthesis pathway upstream of HMG CoA reductase - the molecular targets of statins.[42]
- Activation of adenosine monophosphate activated protein kinase (AMP-activated protein kinase) - AMP-activated protein kinase is a functional enzyme present in the liver, striated muscle, and the brain. It plays a key role in cellular energy homeostasis. It acts as a sensor of the energy-depleted form of ATP (i.e., AMP), and its activation results in stimulation of hepatic fatty acid oxidation and ketogenesis, inhibition of cholesterol synthesis, lipogenesis, and triglyceride synthesis, inhibition of adipocyte lipolysis and lipogenesis, stimulation of skeletal muscle fatty acid oxidation and muscle glucose uptake,[43] and modulation of insulin secretion by pancreatic beta-cells.[44]
In a phase II clinical trial involving 177 patients, ETC-1002 was shown to have a dose-dependent reduction of up to 27% in LDL-C (compared with placebo) observed with the maximum dose (120mg), devoid of serious adverse effects.[42] This approach may represent a new target mechanism to reducing LDL-C, but additional studies are required to determine the safety due to its high possibility of producing similar adverse effects as statins.
Inhibition of SREBP-1
Sterol regulatory element binding proteins (SREBPs) are transcription factors required in the activation of genes involved in cholesterol and fatty acid biosynthesis. Fatostatin, a diarylthiazole derivative, was observed to impair the activation of SREBPs, thereby decreasing the transcription of lipogenic genes in cells.[45] More studies are required regarding the efficacy of this potential target in reducing circulating LDL-C since it also induces the expression of PCSK9 - a serine protease which promotes degradation of LDLR, thereby preventing the clearing of LDL particles from the plasma.
Summary Table
| Class | Drug Company | Agent Name | Mechanism of Action | Efficacy on Lowering LDL-C | Route of Administration | Adverse Effects | Published Clinical Trials |
|---|---|---|---|---|---|---|---|
| Inhibition of Apo B/Antisense oligonucleotides | ISIS Pharmaceuticals | ISIS-301012 or Mipomersen | Inhibits apo B mRNA gene expression | Up to 50% reduction | Subcutaneous injection (SC) | Injection site reactions, flu-like illness, 3-fold asymptomatic elevation of liver transaminases | I, II, III |
| PCSK9 Inhibition | Merck, Sanofi-Aventis–Regeneron, Pfizer–Rinat, Amgen, Santaris, Alnylam, Medicines company, ISIS, Adnexus, Roche/Genentech, Novartis | AMG 145, SAR236553/REGN727, RN316, 1D05-IgG2, RG7652, LGT-209, 1B20, J10, J16, J17 | Inhibition of PCSK9 which promotes degradation of LDLR | 38% reduction in LDL-C in animal studies | ASO (SC), monoclonal antibody (SC), SiRNA (IV) | Phases I, II | |
| MTP Inhibition | Aegerion Pharmaceuticals, Pfizer, Surface Logix | Intestinal non-specific (lometapide, implitapide, CP-34086); Intestinal-specific (SLx-4090, dirlotapide, JTT-130) | Inhibits Microsomal triglyceride transfer protein | Lometapide (up to 50%), CP-34086 (up to 70%) | PO | Intestinal non-specific agents causes GI adverse effects, increases in hepatic fat | Lometapide (I, II, III), JTT-130 (I, II) |
| Thyromimetics | Karo Bio AB | DITPA, eprotirome, sobetirome (GC-1), MB07811, KB-141, T-0681 | TRβ1-selective | Up to 32% reduction of LDL-C | PO | Adverse cardiac effects | Phase II |
| Squalene Synthase Inhibitors | Takeda | TAK-475 | Inhibits squalene synthase in the HMG CoA-Mevalonate pathway | Up to 27% reduction in LDL-C | Phase II | ||
| Inhibition of ACL and Activation of AMPK | Esperion | ETC-1002 | Regulation of lipid and carbohydrate metabolic subtrates | Up to 27% reduction in LDL-C | No serious adverse effects | Phase II | |
| Inhibition of SREBP-1 | Inhibition of SREBP-1 | Impair the activation of SREBPs, thereby decreasing the transcription of lipogenic genes in cells |
References
- ↑ Go, AS.; Mozaffarian, D.; Roger, VL.; Benjamin, EJ.; Berry, JD.; Borden, WB.; Bravata, DM.; Dai, S.; Ford, ES. (2013). "Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association". Circulation. 127 (1): 143–52. doi:10.1161/CIR.0b013e318282ab8f. PMID 23283859. Unknown parameter
|month=ignored (help) - ↑ Heidenreich, PA.; Trogdon, JG.; Khavjou, OA.; Butler, J.; Dracup, K.; Ezekowitz, MD.; Finkelstein, EA.; Hong, Y.; Johnston, SC. (2011). "Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association". Circulation. 123 (8): 933–44. doi:10.1161/CIR.0b013e31820a55f5. PMID 21262990. Unknown parameter
|month=ignored (help) - ↑ Cohen, JC.; Boerwinkle, E.; Mosley, TH.; Hobbs, HH. (2006). "Sequence variations in PCSK9, low LDL, and protection against coronary heart disease". N Engl J Med. 354 (12): 1264–72. doi:10.1056/NEJMoa054013. PMID 16554528. Unknown parameter
|month=ignored (help) - ↑ Yan, AT.; Yan, RT.; Tan, M.; Hackam, DG.; Leblanc, KL.; Kertland, H.; Tsang, JL.; Jaffer, S.; Kates, ML. (2006). "Contemporary management of dyslipidemia in high-risk patients: targets still not met". Am J Med. 119 (8): 676–83. doi:10.1016/j.amjmed.2005.11.015. PMID 16887414. Unknown parameter
|month=ignored (help) - ↑ van der Steeg, WA.; Boekholdt, SM.; Stein, EA.; El-Harchaoui, K.; Stroes, ES.; Sandhu, MS.; Wareham, NJ.; Jukema, JW.; Luben, R. (2007). "Role of the apolipoprotein B-apolipoprotein A-I ratio in cardiovascular risk assessment: a case-control analysis in EPIC-Norfolk". Ann Intern Med. 146 (9): 640–8. PMID 17470832. Unknown parameter
|month=ignored (help) - ↑ Hussain, MM.; Strickland, DK.; Bakillah, A. (1999). "The mammalian low-density lipoprotein receptor family". Annu Rev Nutr. 19: 141–72. doi:10.1146/annurev.nutr.19.1.141. PMID 10448520.
- ↑ Humphries, SE.; Whittall, RA.; Hubbart, CS.; Maplebeck, S.; Cooper, JA.; Soutar, AK.; Naoumova, R.; Thompson, GR.; Seed, M. (2006). "Genetic causes of familial hypercholesterolaemia in patients in the UK: relation to plasma lipid levels and coronary heart disease risk". J Med Genet. 43 (12): 943–9. doi:10.1136/jmg.2006.038356. PMID 17142622. Unknown parameter
|month=ignored (help) - ↑ Marsh, JB.; Welty, FK.; Lichtenstein, AH.; Lamon-Fava, S.; Schaefer, EJ. (2002). "Apolipoprotein B metabolism in humans: studies with stable isotope-labeled amino acid precursors". Atherosclerosis. 162 (2): 227–44. PMID 11996942. Unknown parameter
|month=ignored (help) - ↑ Schonfeld, G.; Lin, X.; Yue, P. (2005). "Familial hypobetalipoproteinemia: genetics and metabolism". Cell Mol Life Sci. 62 (12): 1372–8. doi:10.1007/s00018-005-4473-0. PMID 15818469. Unknown parameter
|month=ignored (help) - ↑ Bennett, CF.; Swayze, EE. (2010). "RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform". Annu Rev Pharmacol Toxicol. 50: 259–93. doi:10.1146/annurev.pharmtox.010909.105654. PMID 20055705.
- ↑ Ito, MK. (2007). "ISIS 301012 gene therapy for hypercholesterolemia: sense, antisense, or nonsense?". Ann Pharmacother. 41 (10): 1669–78. doi:10.1345/aph.1K065. PMID 17848425. Unknown parameter
|month=ignored (help) - ↑ Kastelein, JJ.; Wedel, MK.; Baker, BF.; Su, J.; Bradley, JD.; Yu, RZ.; Chuang, E.; Graham, MJ.; Crooke, RM. (2006). "Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B.". Circulation. 114 (16): 1729–35. doi:10.1161/CIRCULATIONAHA.105.606442. PMID 17030687. Unknown parameter
|month=ignored (help) - ↑ Akdim, F.; Visser, ME.; Tribble, DL.; Baker, BF.; Stroes, ES.; Yu, R.; Flaim, JD.; Su, J.; Stein, EA. (2010). "Effect of mipomersen, an apolipoprotein B synthesis inhibitor, on low-density lipoprotein cholesterol in patients with familial hypercholesterolemia". Am J Cardiol. 105 (10): 1413–9. doi:10.1016/j.amjcard.2010.01.003. PMID 20451687. Unknown parameter
|month=ignored (help) - ↑ Akdim, F.; Tribble, DL.; Flaim, JD.; Yu, R.; Su, J.; Geary, RS.; Baker, BF.; Fuhr, R.; Wedel, MK. (2011). "Efficacy of apolipoprotein B synthesis inhibition in subjects with mild-to-moderate hyperlipidaemia". Eur Heart J. 32 (21): 2650–9. doi:10.1093/eurheartj/ehr148. PMID 21593041. Unknown parameter
|month=ignored (help) - ↑ Raal, FJ.; Santos, RD.; Blom, DJ.; Marais, AD.; Charng, MJ.; Cromwell, WC.; Lachmann, RH.; Gaudet, D.; Tan, JL. (2010). "Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolaemia: a randomised, double-blind, placebo-controlled trial". Lancet. 375 (9719): 998–1006. doi:10.1016/S0140-6736(10)60284-X. PMID 20227758. Unknown parameter
|month=ignored (help) - ↑ *"The Evolving Role of PCSK9 Modulation in the Regulation of LDL-Cholesterol". 2012-11-11.
- ↑ Maxwell, KN.; Soccio, RE.; Duncan, EM.; Sehayek, E.; Breslow, JL. (2003). "Novel putative SREBP and LXR target genes identified by microarray analysis in liver of cholesterol-fed mice". J Lipid Res. 44 (11): 2109–19. doi:10.1194/jlr.M300203-JLR200. PMID 12897189. Unknown parameter
|month=ignored (help) - ↑ Le May, C.; Kourimate, S.; Langhi, C.; Chétiveaux, M.; Jarry, A.; Comera, C.; Collet, X.; Kuipers, F.; Krempf, M. (2009). "Proprotein convertase subtilisin kexin type 9 null mice are protected from postprandial triglyceridemia". Arterioscler Thromb Vasc Biol. 29 (5): 684–90. doi:10.1161/ATVBAHA.108.181586. PMID 19265033. Unknown parameter
|month=ignored (help) - ↑ Sun, H.; Samarghandi, A.; Zhang, N.; Yao, Z.; Xiong, M.; Teng, BB. (2012). "Proprotein convertase subtilisin/kexin type 9 interacts with apolipoprotein B and prevents its intracellular degradation, irrespective of the low-density lipoprotein receptor". Arterioscler Thromb Vasc Biol. 32 (7): 1585–95. doi:10.1161/ATVBAHA.112.250043. PMID 22580899. Unknown parameter
|month=ignored (help) - ↑ Wetterau, JR.; Lin, MC.; Jamil, H. (1997). "Microsomal triglyceride transfer protein". Biochim Biophys Acta. 1345 (2): 136–50. PMID 9106493. Unknown parameter
|month=ignored (help) - ↑ Sharp, D.; Blinderman, L.; Combs, KA.; Kienzle, B.; Ricci, B.; Wager-Smith, K.; Gil, CM.; Turck, CW.; Bouma, ME. (1993). "Cloning and gene defects in microsomal triglyceride transfer protein associated with abetalipoproteinaemia". Nature. 365 (6441): 65–9. doi:10.1038/365065a0. PMID 8361539. Unknown parameter
|month=ignored (help) - ↑ Rader, DJ.; Brewer, HB. (1993). "Abetalipoproteinemia. New insights into lipoprotein assembly and vitamin E metabolism from a rare genetic disease". JAMA. 270 (7): 865–9. PMID 8340987. Unknown parameter
|month=ignored (help) - ↑ Cuchel, M.; Bloedon, LT.; Szapary, PO.; Kolansky, DM.; Wolfe, ML.; Sarkis, A.; Millar, JS.; Ikewaki, K.; Siegelman, ES. (2007). "Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia". N Engl J Med. 356 (2): 148–56. doi:10.1056/NEJMoa061189. PMID 17215532. Unknown parameter
|month=ignored (help) - ↑ Samaha, FF.; McKenney, J.; Bloedon, LT.; Sasiela, WJ.; Rader, DJ. (2008). "Inhibition of microsomal triglyceride transfer protein alone or with ezetimibe in patients with moderate hypercholesterolemia". Nat Clin Pract Cardiovasc Med. 5 (8): 497–505. doi:10.1038/ncpcardio1250. PMID 18506154. Unknown parameter
|month=ignored (help) - ↑ Chandler, CE.; Wilder, DE.; Pettini, JL.; Savoy, YE.; Petras, SF.; Chang, G.; Vincent, J.; Harwood, HJ. (2003). "CP-346086: an MTP inhibitor that lowers plasma cholesterol and triglycerides in experimental animals and in humans". J Lipid Res. 44 (10): 1887–901. doi:10.1194/jlr.M300094-JLR200. PMID 12837854. Unknown parameter
|month=ignored (help) - ↑ Li, J.; Bronk, BS.; Dirlam, JP.; Blize, AE.; Bertinato, P.; Jaynes, BH.; Hickman, A.; Miskell, C.; Pillai, UA. (2007). "In vitro and in vivo profile of 5-[(4'-trifluoromethyl-biphenyl-2-carbonyl)-amino]-1H-indole-2-carboxylic acid benzylmethyl carbamoylamide (dirlotapide), a novel potent MTP inhibitor for obesity". Bioorg Med Chem Lett. 17 (7): 1996–9. doi:10.1016/j.bmcl.2007.01.018. PMID 17276061. Unknown parameter
|month=ignored (help) - ↑ STRISOWER, B.; GOFMAN, JW.; GALIONI, EF.; ALMADA, AA.; SIMON, A. (1954). "Effect of thyroid extract on serum lipoproteins and serum cholesterol". Metabolism. 3 (3): 218–27. PMID 13165045. Unknown parameter
|month=ignored (help) - ↑ STRISOWER, B.; ELMLINGER, P.; GOFMAN, JW.; DELALLA, O. (1959). "The effect of 1-thyroxine on serum lipoprotein and cholesterol concentrations". J Clin Endocrinol Metab. 19 (1): 117–26. PMID 13620737. Unknown parameter
|month=ignored (help) - ↑ HOLLISTER, LE.; ARONS, WL. (1962). "Effect of dextro-isomers of thyroid hormones on serum cholesterol levels in euthyroid hypercholesterolemic patients". Ann Intern Med. 56: 570–6. PMID 13908447. Unknown parameter
|month=ignored (help) - ↑ Brown, MS.; Goldstein, JL. (1986). "A receptor-mediated pathway for cholesterol homeostasis". Science. 232 (4746): 34–47. PMID 3513311. Unknown parameter
|month=ignored (help) - ↑ Young, WF.; Gorman, CA.; Jiang, NS.; Machacek, D.; Hay, ID. (1984). "L-thyroxine contamination of pharmaceutical D-thyroxine: probable cause of therapeutic effect". Clin Pharmacol Ther. 36 (6): 781–7. PMID 6499357. Unknown parameter
|month=ignored (help) - ↑ 32.0 32.1 Angelin, B.; Rudling, M. (2010). "Lipid lowering with thyroid hormone and thyromimetics". Curr Opin Lipidol. 21 (6): 499–506. doi:10.1097/MOL.0b013e3283402e9c. PMID 20935564. Unknown parameter
|month=ignored (help) - ↑ Gullberg, H.; Rudling, M.; Saltó, C.; Forrest, D.; Angelin, B.; Vennström, B. (2002). "Requirement for thyroid hormone receptor beta in T3 regulation of cholesterol metabolism in mice". Mol Endocrinol. 16 (8): 1767–77. PMID 12145333. Unknown parameter
|month=ignored (help) - ↑ Johansson, C.; Vennström, B.; Thorén, P. (1998). "Evidence that decreased heart rate in thyroid hormone receptor-alpha1-deficient mice is an intrinsic defect". Am J Physiol. 275 (2 Pt 2): R640–6. PMID 9688704. Unknown parameter
|month=ignored (help) - ↑ Erion, MD.; Cable, EE.; Ito, BR.; Jiang, H.; Fujitaki, JM.; Finn, PD.; Zhang, BH.; Hou, J.; Boyer, SH. (2007). "Targeting thyroid hormone receptor-beta agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index". Proc Natl Acad Sci U S A. 104 (39): 15490–5. doi:10.1073/pnas.0702759104. PMID 17878314. Unknown parameter
|month=ignored (help) - ↑ Tancevski, I.; Demetz, E.; Eller, P.; Duwensee, K.; Hoefer, J.; Heim, C.; Stanzl, U.; Wehinger, A.; Auer, K. (2010). "The liver-selective thyromimetic T-0681 influences reverse cholesterol transport and atherosclerosis development in mice". PLoS One. 5 (1): e8722. doi:10.1371/journal.pone.0008722. PMID 20090943.
- ↑ Ladenson, PW.; Kristensen, JD.; Ridgway, EC.; Olsson, AG.; Carlsson, B.; Klein, I.; Baxter, JD.; Angelin, B. (2010). "Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia". N Engl J Med. 362 (10): 906–16. doi:10.1056/NEJMoa0905633. PMID 20220185. Unknown parameter
|month=ignored (help) - ↑ Tavridou, A.; Kaklamanis, L.; Megaritis, G.; Kourounakis, AP.; Papalois, A.; Roukounas, D.; Rekka, EA.; Kourounakis, PN.; Charalambous, A. (2006). "Pharmacological characterization in vitro of EP2306 and EP2302, potent inhibitors of squalene synthase and lipid biosynthesis". Eur J Pharmacol. 535 (1–3): 34–42. doi:10.1016/j.ejphar.2006.02.006. PMID 16545796. Unknown parameter
|month=ignored (help) - ↑ Mühlhäuser, U.; Zolk, O.; Rau, T.; Münzel, F.; Wieland, T.; Eschenhagen, T. (2006). "Atorvastatin desensitizes beta-adrenergic signaling in cardiac myocytes via reduced isoprenylation of G-protein gamma-subunits". FASEB J. 20 (6): 785–7. doi:10.1096/fj.05-5067fje. PMID 16467371. Unknown parameter
|month=ignored (help) - ↑ Nishimoto, T.; Ishikawa, E.; Anayama, H.; Hamajyo, H.; Nagai, H.; Hirakata, M.; Tozawa, R. (2007). "Protective effects of a squalene synthase inhibitor, lapaquistat acetate (TAK-475), on statin-induced myotoxicity in guinea pigs". Toxicol Appl Pharmacol. 223 (1): 39–45. doi:10.1016/j.taap.2007.05.005. PMID 17599378. Unknown parameter
|month=ignored (help) - ↑ Pinkosky, SL.; Filippov, S.; Srivastava, RA.; Hanselman, JC.; Bradshaw, CD.; Hurley, TR.; Cramer, CT.; Spahr, MA.; Brant, AF. (2013). "AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism". J Lipid Res. 54 (1): 134–51. doi:10.1194/jlr.M030528. PMID 23118444. Unknown parameter
|month=ignored (help) - ↑ 42.0 42.1 Ballantyne, CM.; Davidson, MH.; Macdougall, DE.; Bays, HE.; Dicarlo, LA.; Rosenberg, NL.; Margulies, J.; Newton, RS. (2013). "Efficacy and safety of a novel dual modulator of adenosine triphosphate-citrate lyase and adenosine monophosphate-activated protein kinase in patients with hypercholesterolemia: results of a multicenter, randomized, double-blind, placebo-controlled, parallel-group trial". J Am Coll Cardiol. 62 (13): 1154–62. doi:10.1016/j.jacc.2013.05.050. PMID 23770179. Unknown parameter
|month=ignored (help) - ↑ Holmes, BF.; Kurth-Kraczek, EJ.; Winder, WW. (1999). "Chronic activation of 5'-AMP-activated protein kinase increases GLUT-4, hexokinase, and glycogen in muscle". J Appl Physiol (1985). 87 (5): 1990–5. PMID 10562646. Unknown parameter
|month=ignored (help) - ↑ Winder, WW.; Hardie, DG. (1999). "AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes". Am J Physiol. 277 (1 Pt 1): E1–10. PMID 10409121. Unknown parameter
|month=ignored (help) - ↑ Kamisuki, S.; Mao, Q.; Abu-Elheiga, L.; Gu, Z.; Kugimiya, A.; Kwon, Y.; Shinohara, T.; Kawazoe, Y.; Sato, S. (2009). "A small molecule that blocks fat synthesis by inhibiting the activation of SREBP". Chem Biol. 16 (8): 882–92. doi:10.1016/j.chembiol.2009.07.007. PMID 19716478. Unknown parameter
|month=ignored (help)