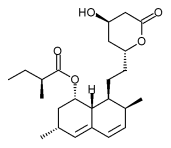
Lovastatin
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | <5% |
| Protein binding | >95% |
| Metabolism | hepatic (CYP3A substrate) |
| Elimination half-life | 1.1-1.7 hours |
| Excretion | negligible |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C24H36O5 |
| Molar mass | 404.54 g/mol |
|
WikiDoc Resources for Lovastatin |
|
Articles |
|---|
|
Most recent articles on Lovastatin |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Lovastatin at Clinical Trials.gov Clinical Trials on Lovastatin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Lovastatin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Lovastatin Discussion groups on Lovastatin Patient Handouts on Lovastatin Directions to Hospitals Treating Lovastatin Risk calculators and risk factors for Lovastatin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Lovastatin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
| Cardiology Network |
 Discuss Lovastatin further in the WikiDoc Cardiology Network |
| Adult Congenital |
|---|
| Biomarkers |
| Cardiac Rehabilitation |
| Congestive Heart Failure |
| CT Angiography |
| Echocardiography |
| Electrophysiology |
| Cardiology General |
| Genetics |
| Health Economics |
| Hypertension |
| Interventional Cardiology |
| MRI |
| Nuclear Cardiology |
| Peripheral Arterial Disease |
| Prevention |
| Public Policy |
| Pulmonary Embolism |
| Stable Angina |
| Valvular Heart Disease |
| Vascular Medicine |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [3] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
For patient information, click here
Overview
Lovastatin is a member of the drug class of statins, used for lowering cholesterol (hypolipidemic agent) in those with hypercholesterolemia and so preventing cardiovascular disease.
History
Lovastatin was isolated from a strain of Aspergillus terreus and it was the first statin approved by the FDA (August 1987).
Lovastatin is also naturally produced by certain higher fungi such as Pleurotus ostreatus (oyster mushroom) and closely related Pleurotus spp.[1]
In 1998, the US Food and Drug Administration (FDA) placed a ban on the sale of dietary supplements derived from red yeast rice, which naturally contains lovastatin, arguing that products containing prescription agents require drug approval.
Compactin and lovastatin, natural products with a powerful inhibitory effect on HMG-CoA reductase, were discovered in the 1970s, and taken into clinical development as potential drugs for lowering LDL cholesterol. [2]
However, in 1980, trials with compactin were suspended for undisclosed reasons (rumoured to be related to serious animal toxicity). Because of the close structural similarity between compactin and lovastatin, clinical studies with lovastatin were also suspended, and additional animal safety studies initiated.
In 1982 some small-scale clinical investigations of lovastatin, a polyketide-derived natural product isolated from Aspergillus terreus, in very high-risk patients were undertaken, in which dramatic reductions in LDL cholesterol were observed, with very few adverse effects. After the additional animal safety studies with lovastatin revealed no toxicity of the type thought to be associated with compactin, clinical studies resumed.
Large-scale trials confirmed the effectiveness of lovastatin. Observed tolerability continued to be excellent, and lovastatin was approved by the US FDA in 1987.
Lovastatin at its maximal recommended dose of 80 mg daily produced a mean reduction in LDL cholesterol of 40%, a far greater reduction than could be obtained with any of the treatments available at the time. Equally important, the drug produced very few adverse effects, was easy for patients to take, and so was rapidly accepted by prescribers and patients. The only important adverse effect is myopathy/rhabdomyolysis. This is rare and occurs with all HMG-CoA reductase inhibitors.
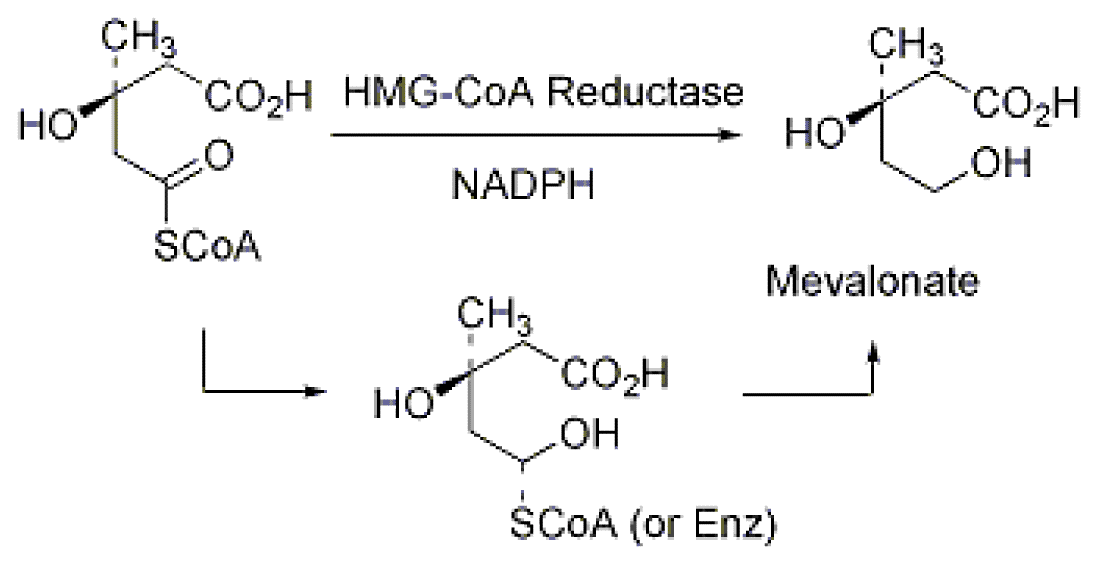
Mechanism of action
Lovastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase), an enzyme which catalyzes the conversion of HMG-CoA to mevalonate .[3] Mevalonate is a required building block for cholesterol biosynthesis and lovastatin interferes with its production by acting as a competitive inhibitor for HMG-CoA which binds to the HMG-CoA reductase. Lovastatin, being inactive in the native form, the form in which it is administered, is hydrolysed to the β-hydroxy acid form in the body and it is this form which is active. Presumably, the reductase acts on the hydrolyzed lovastatin to reduce the carboxylic acid moiety.
Discovery, biochemistry and biology
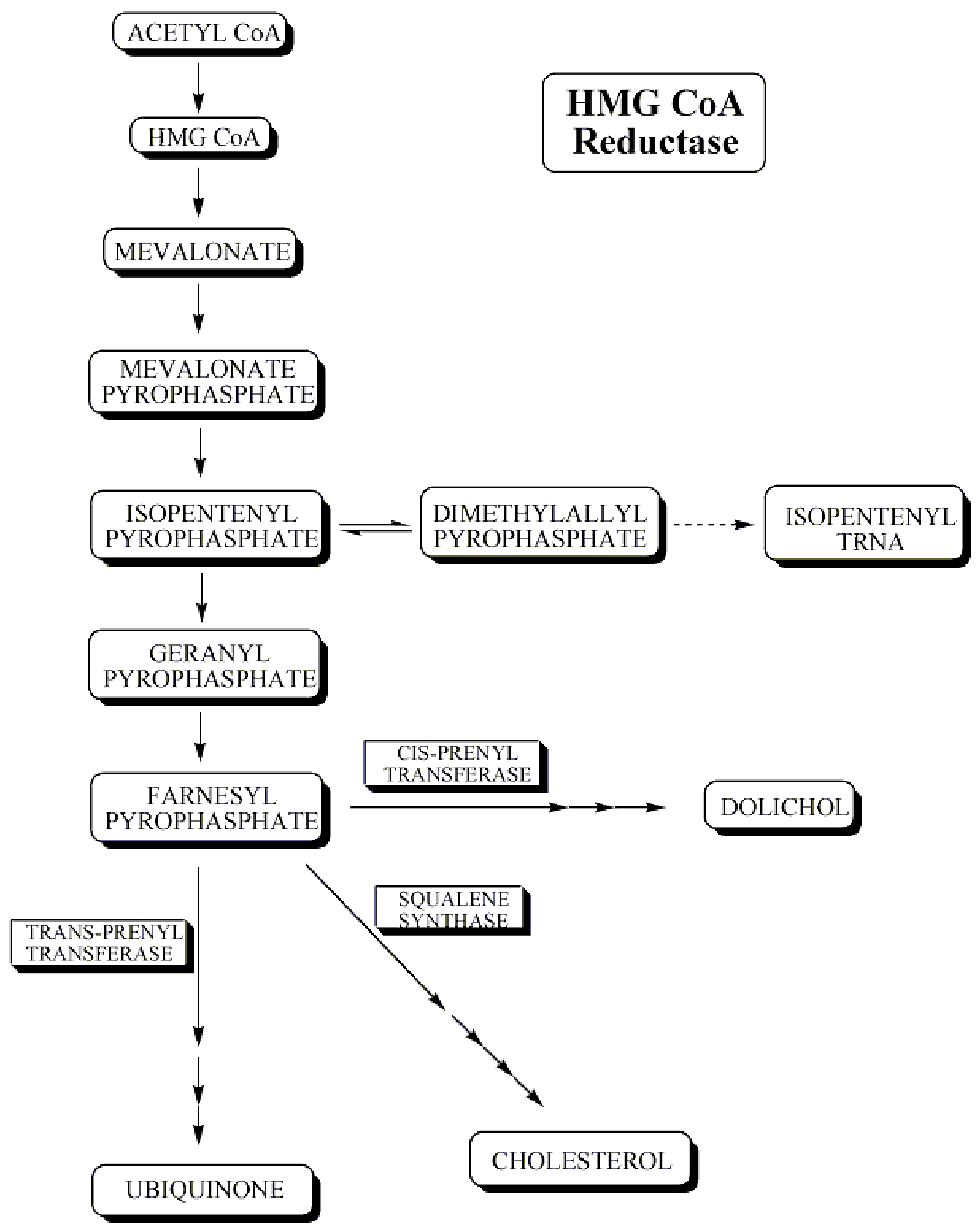
It is now generally accepted that a major risk factor for the development of coronary artery disease is an elevated concentration of plasma cholesterol, especially low density lipoprotein (LDL) cholesterol. The objective is to decrease excess levels of cholesterol to an amount consistent with maintenance of normal body function. Cholesterol is biosynthesized in a series of more than 25 separate enzymatic reactions that initially involves 3 successive condensations of acetyl-CoA units to form a 6-carbon compound, 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA). This is reduced to mevalonate and then converted in a series of reactions to the isoprenes that are building blocks of squalene, the immediate precursor to sterols, which cyclizes to lanosterol (a methylated sterol) and further metabolized to cholesterol.
A number of early attempts to block the synthesis of cholesterol resulted in agents that inhibited late in the biosynthetic pathway between lanosterol and cholesterol. A major rate limiting step in the pathway is at the level of the microsomal enzyme which catalyzes the conversion of HMG CoA to mevalonic acid and which has been considered to be a prime target for pharmacologic intervention for several years.[4]
HMG CoA reductase occurs early in the biosynthetic pathway and is among the first committed steps to cholesterol formulation. Inhibition of this enzyme could lead to accumulation of HMG CoA, a water-soluble intermediate that is then capable of being readily metabolized to simpler molecules. This inhibition of reductase would lead to accumulation of lipophylic intermediates having a formal sterol ring.
Lovastatin is the first specific inhibitor of HMG CoA reductase to receive approval for the treatment of hypercholesterolemia. The first breakthrough in efforts to find a potent, specific, competitive inhibitor of HMG CoA reductase occurred in 1976 when Endo et al reported discovery of mevastatin, a highly functionalized fungal metabolite, isolated from cultures of Penicillium citrium. Mevastatin was demonstrated to be an unusually potent inhibitor of the target enzyme and of cholesterol biosynthesis. Subsequent to the first reports describing mevastatin, efforts were initiated to search for other naturally occurring inhibitors oh HMG CoA reductase. This led to the discovery of a novel fungal metabolite – Lovastatin. The structure of Lovastatin was determined to be different from that of mevastatin by the presence of a 6 alphamethyl group in the hexahydronaphthalene ring.
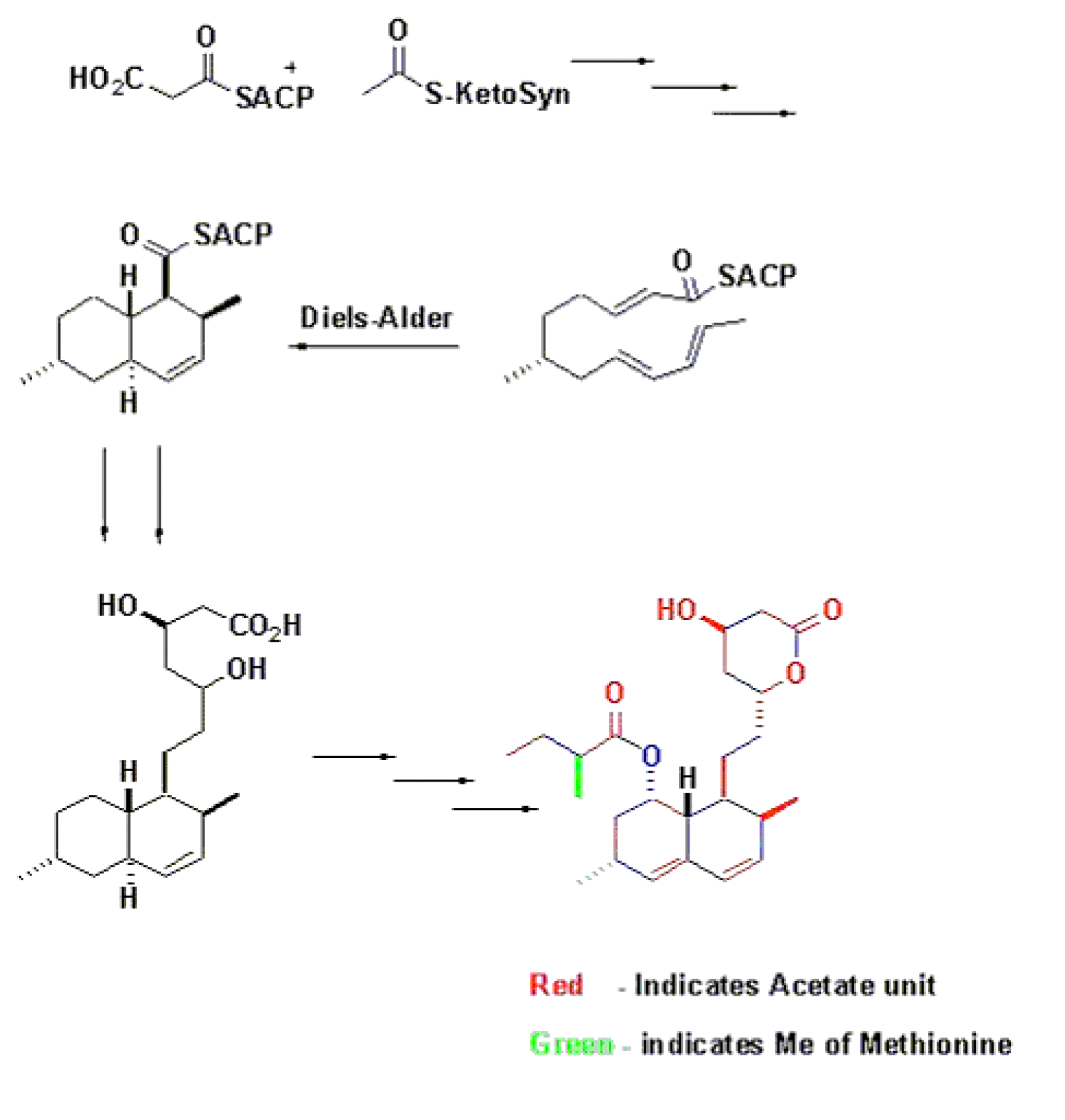
Key points from the study of the Biosynthesis of Lovastatin :-
Lovastatin is comprised of 2 polyketide chains derived from acetate that are 8- and 4- carbons long coupled in head to tail fashion.
6 alphamethyl group and the methyl group on the 4-carbon side chain are derived from the methyl group of methionine, and
6 alphamethyl group is added before closure of the rings.
This implies that lovastatin is a unique compound synthesized by A. terreus and that mevastatin is not an intermediate in its formation.
Cholesterol biosynthetic pathway
HMG CoA reductase reaction
Biosynthesis -- Diels-Alder catalyzed cyclization
In vitro formation of a triketide lactone using a genetically-modified protein derived from 6-deoxyerythronolide B synthase has been demonstrated.[5] The stereochemistry of the molecule supports the intriguing idea that an enzyme-catalyzed Diels-Alder reaction may occur during assembly of the polyketide chain. It thus appears that biological Diels-Alder reactions may be triggered by generation of reactive triene systems on an enzyme surface.
Biosynthesis – Using Broadly specific Acyltransferase
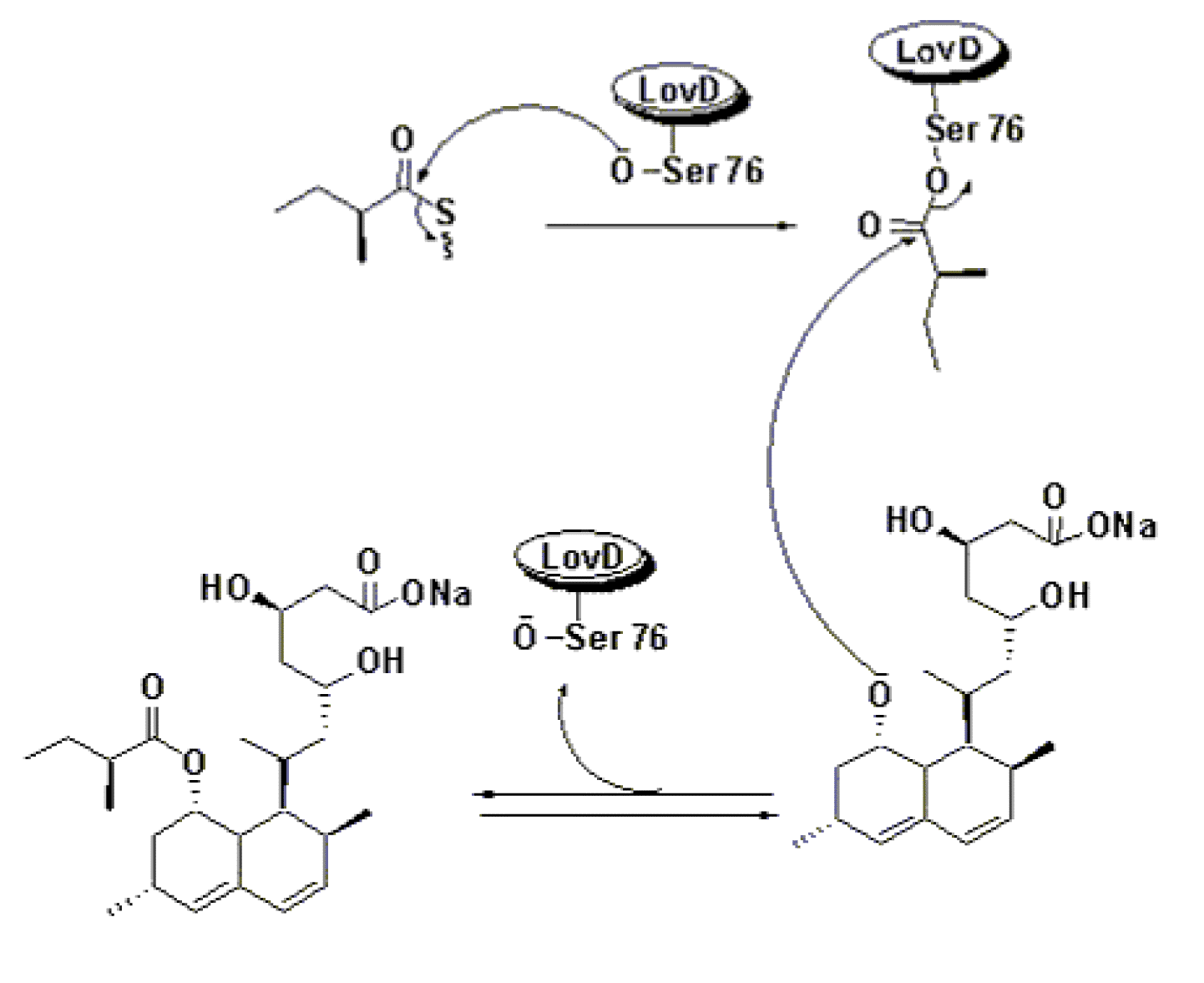
It has been found that a dedicated acyltransferase, LovD, is encoded in the lovastatin biosynthetic pathway. LovD has a broad substrate specificity towards the acyl carrier, the acyl substrate and the decalin acyl acceptor. It efficiently catalyzes the acyl transfer from coenzyme A thoesters or N-acetylcysteamine (SNAC) thioesters to monacolin J.[6]
The biosynthesis of Lovastatin is coordinated by two iterative type I polyketide syntheses and numerous accessory enzymes. Nonketide, the intermediate biosynthetic precursor of Lovastatin, is assembled by the upstream megasynthase LovB (also known as lovastatin nonaketide synthase), enoylreductase LovC, and CYP450 oxygenases. The five carbon unit side chain is synthesized by LovF (also known as lovastatin diketide synthase) through a single condensation diketide undergoes methylation and reductive tailoring by the individual LovF catalytic domains to yield an α-S-methylbutyryl thioester covalently attached to the phosphopantetheine arm on the acyl carrier protein (ACP) domain of LovF. Encoded in the gene cluster is a 46kDa protein, LovD, which was initially identified as an esterase homolog. LovD, which was initially identified as an esterase homolog. LovD was suggested to catalyze the last step of lovastatin biosynthesis that regioselectively transacylates the acyl group from LovF to the C8 hydroxyl group of the Nonaketide to yield Lovastatin.
Total synthesis
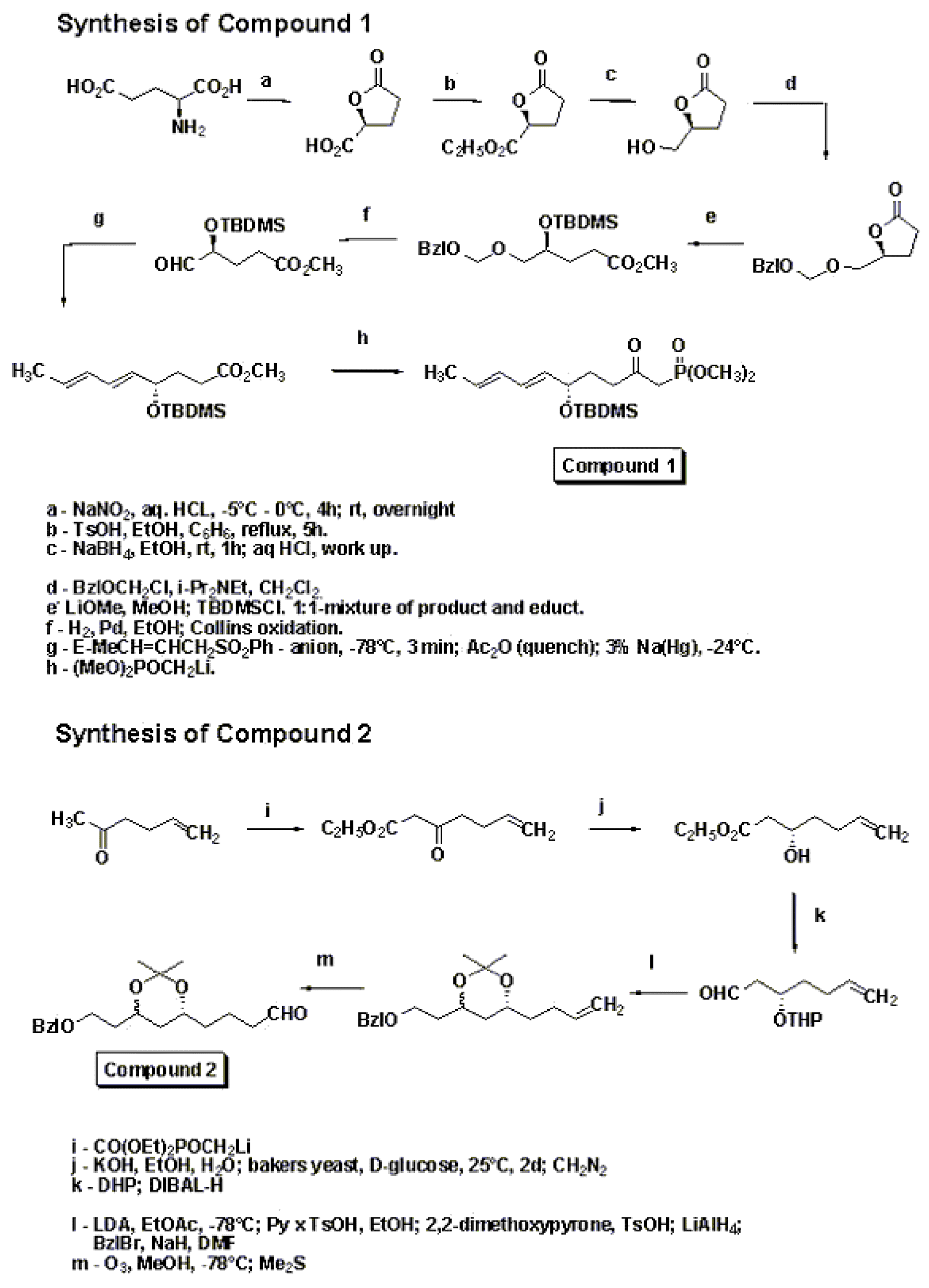
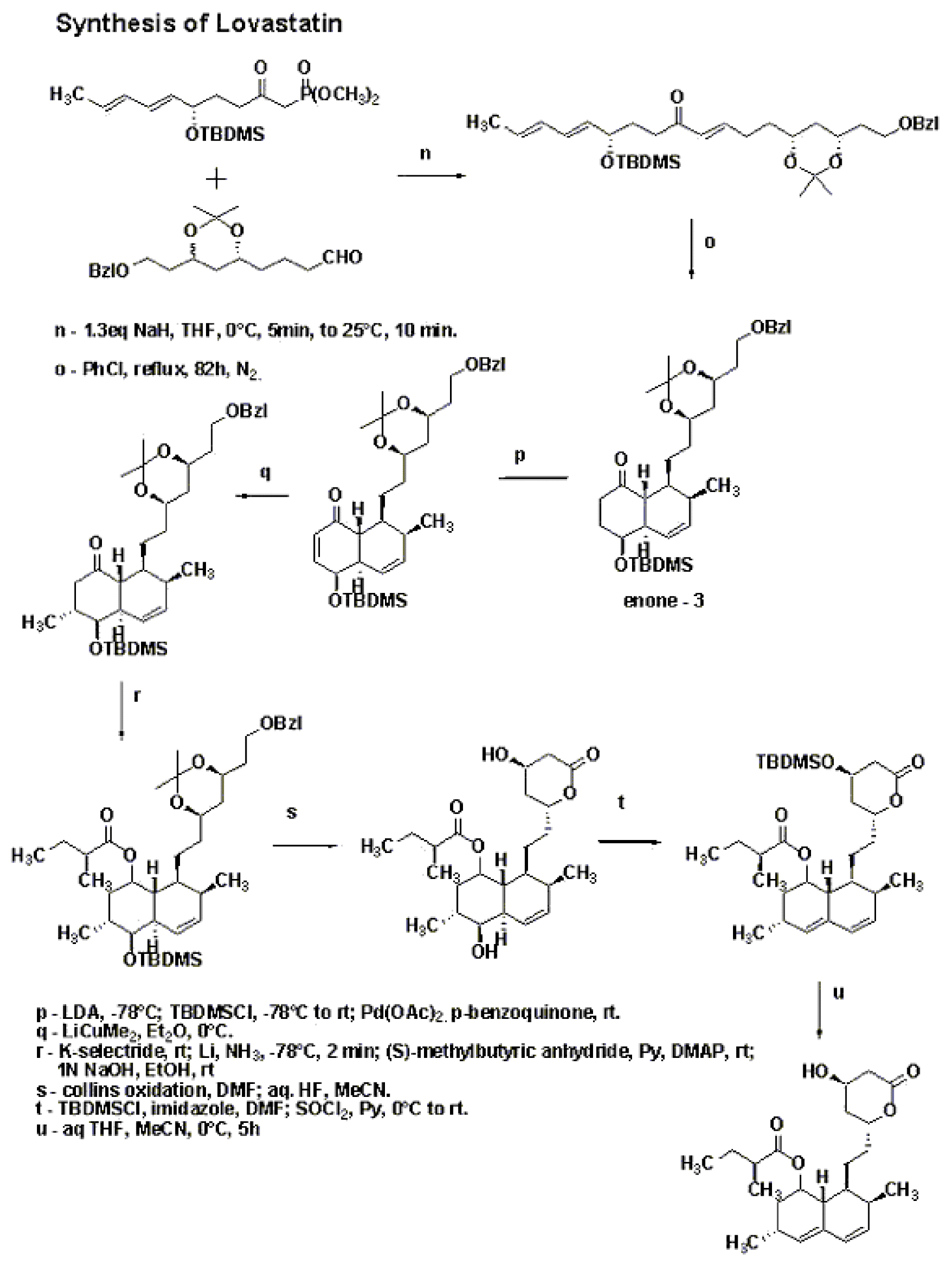
A major bulk of work in the synthesis of Lovastatin was done by M. Hirama in the 1980’s.[7] [8] Hirama synthesized Compactin and used one of the intermediates to follow a different path to get to Lovastatin. The synthetic sequence is shown in the schemes below. The γ-lactone was synthesized using Yamada methodology starting with aspartic acid. Lactone opening was done using lithium methoxide in methanol and then silylation to give a separable mixture of the starting lactone and the silyl ether. The silyl ether on hydrogenolysis followed by Collins oxidation gave the aldehyde. Stereoselective preparation of (E,E)-diene was accomplished by addition of trans-crotyl phenyl sulfone anion, followed by quenching with Ac2O and subsequent reductive elimination of sulfone acetate. Condensation of this with Lithium anion of dimethyl methylphosphonate gave compound 1.Compound 2 was synthesized as shown in the scheme in the synthetic procedure. Compounds 1 and 2 were then combined together using 1.3eq sodium hydride in THF followed by reflux in chlorobenzene for 82 hrs under nitrogen to get the enone 3.
Simple organic reactions were used to get to Lovastatin as shown in the scheme.
Synthesis of compounds 1 and 2
Synthesis of lovastatin
Pharmacology and dose
The mode of action of statins is HMG-CoA reductase enzyme inhibition. This enzyme is needed by the body to make cholesterol.
Lovastatin causes cholesterol to be lost from LDL, but also reduces the concentration of circulating LDL (low density lipoprotein) particles. Apolipoprotein B concentration falls substantially during treatment with lovastatin. Lovastatin's ability to lower LDL is thought to be due to a reduction in VLDL, which is a precursor to LDL. Also, Lovastatin may increase the number of LDL receptors on the surface of cell membranes, and thus increase the breakdown of LDL.
Lovastatin can also produce slight to moderate increases in HDL, and slight to moderate decreases in triglycerides. Both of these effects are typically beneficial to a patient with a poor lipid profile.
Both lovastatin and its b-hydroxyacid metabolite are highly bound (>95%) to human plasma proteins. Animal studies demonstrated that lovastatin crosses the blood-brain and placental barriers.[9] Elderly patients, or those with renal insufficiency may have higher plasma concentrations of lovastatin after administration and may require a lower dose. The usual recommended starting dose is 20 mg once a day given with the evening meal, and the dose range is 10-80 mg a day in a single dose, or divided into two doses.
Side effects
Lovastatin is usually well tolerated. Lovastatin, and all statin drugs, can rarely cause myopathy or rhabdomyolysis. This can be life-threatening if not recognised and treated in time, and so any unexplained muscle pain or weakness whilst on lovastatin should be promptly mentioned to the prescribing doctor.
Drug interactions
As with all the statin drugs, drinking grapefruit juice during therapy increases the risk of serious side effects. Grapefruit juice inhibits CYP3A4, and thus decreases the metabolism of statins, increasing their plasma concentrations.
Lovastatin at doses higher than 20 mg per day should not be used in conjunction with gemfibrozil or other fibrates, niacin, or cyclosporin. This is because of the significantly increased risk of rhabdomyolysis.
Pharmacopoeia information
Lovastatin tablets are preserved in well closed, light resistant containers. Protected from light and stored either in a cool place or at controlled room temperature.
Lovastatin tablets are tested for Dissolution and Assay as per the USP.
Limit for Dissolution – Not less than 80% (Q) of the labeled amount of Lovastatin is dissolved in 30 mins.
Limit for Assay – Each tablet contains not less than 90% and not more than 110% of the labeled amount of Lovastatin, tested by HPLC analysis.
Lovastatin raw material contains 5 impurities – A, B, C, D and E (as shown below).
Brand names
- Mevacor®
- Advicor® (as a combination with niacin)
- Altocor®
- Altoprev®
- Statosan® (Atos Pharma)
See also
References
- ↑ Bobek P, Ozdín L, Galbavý S (1998). "Dose- and time-dependent hypocholesterolemic effect of oyster mushroom (Pleurotus ostreatus) in rats". Nutrition. 14 (3): 282–6. PMID 9583372.
- ↑ Vederas, J.C,Moore, R. N., Bigam, G., Chan, K. J. (1985). "Biosynthesis of the Hypocholesterolemic agent Mevinolin by Aspergillus terreus. Determination of the origin of carbon, Hydrogen and Oxygen by 13C NMR and mass Spectrometry". 'J. Am. Chem. Soc.'. 107: 3694–3701.
- ↑ Alberts, A. W. (1998). "Discovery, Biochemistry and Biology of Lovastatin". The American Journal of Cardiology. 62: 10J–15J.
- ↑ Alberts, A. W. (1998). "Discovery, Biochemistry and Biology of Lovastatin". The American Journal of Cardiology. 62: 10J–15J.
- ↑ Vederas, J. C., Witter, D. J. (1996). "Putative Diels-Alder Catalyzed Cyclization during the Biosynthesis of Lovastatin". J. Org. Chem. 61 (8): 2613–2623.
- ↑ Tang, Y., Wang, C. C., Watanbe, K., Xie, X., Wojcicki, W. A. (2006). "Biosynthesis of Lovastatin Analogs with Broadly Specific Acyltransferase". Chemistry and Biology. 13: 1161–1169.
- ↑ Hirama, M., Vet, M. (1982). "Synthesis of Compactin starting from Naturally occurring building blocks and using an asymmetry inducing reaction". J. Am. Chem. Soc. 104: 4251.
- ↑ Hirama, M., Iwashita (1983). "Synthesis of (+)-Mevinolin starting from Naturally occurring building blocks and using an asymmetry inducing reaction". Tetrahedron Lett.: 1811–1812.
- ↑ "Lovastatin". Rxlist.com.
- Pages with script errors
- CS1 maint: Multiple names: authors list
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Cardiology
- Statins
- Drugs