Intrauterine device: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
__NOTOC__ | |||
{{BirthControl infobox | | {{BirthControl infobox | | ||
| image = Intrauterine device 001.jpg | | image = Intrauterine device 001.jpg | ||
| Line 45: | Line 46: | ||
The arms of the frame hold the IUD in place near the top of the uterus. The GyneFix does not have a T-shape, but rather is a loop that holds several copper tubes. The GyneFix is held in place by a suture to the [[Fundus (uterus)|fundus of the uterus]]. All copper-containing IUDs have a number as part of their name. This is the surface area of copper (in square millimeters) the IUD provides. | The arms of the frame hold the IUD in place near the top of the uterus. The GyneFix does not have a T-shape, but rather is a loop that holds several copper tubes. The GyneFix is held in place by a suture to the [[Fundus (uterus)|fundus of the uterus]]. All copper-containing IUDs have a number as part of their name. This is the surface area of copper (in square millimeters) the IUD provides. | ||
==Effectiveness and | ==Effectiveness and Mechanism of Contraception== | ||
All second-generation copper-T IUDs have failure rates of less than 1% per year, and cumulative 10-year failure rates of 2-6%.<!-- | All second-generation copper-T IUDs have failure rates of less than 1% per year, and cumulative 10-year failure rates of 2-6%.<!-- | ||
--><ref name="population">IUDs-An Update. [http://www.infoforhealth.org/pr/b6/b6chap2_3.shtml#top Chapter 2.3: Effectiveness].</ref> | --><ref name="population">IUDs-An Update. [http://www.infoforhealth.org/pr/b6/b6chap2_3.shtml#top Chapter 2.3: Effectiveness].</ref> | ||
| Line 115: | Line 116: | ||
--><ref name="pp">{{cite web | title = Understanding IUDs | work = Planned Parenthood Federation of America | date = July 2005 | url = http://www.plannedparenthood.org/pp2/portal/files/portal/medicalinfo/birthcontrol/pub-contraception-iud.xml#1103415754536::8998200633989168060 | accessdate = 2006-07-22 }}</ref> | --><ref name="pp">{{cite web | title = Understanding IUDs | work = Planned Parenthood Federation of America | date = July 2005 | url = http://www.plannedparenthood.org/pp2/portal/files/portal/medicalinfo/birthcontrol/pub-contraception-iud.xml#1103415754536::8998200633989168060 | accessdate = 2006-07-22 }}</ref> | ||
==Side | ==Side Effects and Complications== | ||
Insertion of the IUD may introduce bacteria into the [[uterus]]. The insertion process carries a small, transient increased risk of [[pelvic inflammatory disease]] in the first 20 days following insertion.<!-- | Insertion of the IUD may introduce bacteria into the [[uterus]]. The insertion process carries a small, transient increased risk of [[pelvic inflammatory disease]] in the first 20 days following insertion.<!-- | ||
--><ref name="farley"/> It is very important that the provider use proper [[infection]]-prevention techniques during insertion.<!-- | --><ref name="farley"/> It is very important that the provider use proper [[infection]]-prevention techniques during insertion.<!-- | ||
| Line 136: | Line 137: | ||
--><ref>IUDs-An Update. [http://www.infoforhealth.org/pr/b6/b6chap2_8.shtml#top Chapter 2.8: Intrauterine Pregnancy].</ref> | --><ref>IUDs-An Update. [http://www.infoforhealth.org/pr/b6/b6chap2_8.shtml#top Chapter 2.8: Intrauterine Pregnancy].</ref> | ||
==Use as | ==Use as Emergency Contraception== | ||
Intrauterine devices can be used as [[emergency contraception]] to prevent pregnancy up to 5 days after unprotected [[sexual intercourse]], or sexual intercourse during which the primary contraception is believed to have failed (e.g. a [[condom]] was used, but it broke). Insertion of a copper-T IUD as emergency contraception is more than 99% effective, making it more effective than [[Emergency contraception|emergency contraceptive pills]] ('''ECP''' or 'morning-after pill'). | Intrauterine devices can be used as [[emergency contraception]] to prevent pregnancy up to 5 days after unprotected [[sexual intercourse]], or sexual intercourse during which the primary contraception is believed to have failed (e.g. a [[condom]] was used, but it broke). Insertion of a copper-T IUD as emergency contraception is more than 99% effective, making it more effective than [[Emergency contraception|emergency contraceptive pills]] ('''ECP''' or 'morning-after pill'). | ||
| Line 150: | Line 151: | ||
--><ref name="coils">{{cite web | title = Contraceptive coils (IUDs) | work = NetDoctor.co.uk | date = 2006 | url = http://www.netdoctor.co.uk/sex_relationships/facts/contraceptivecoil.htm | accessdate = 2006-07-05 }}</ref> | --><ref name="coils">{{cite web | title = Contraceptive coils (IUDs) | work = NetDoctor.co.uk | date = 2006 | url = http://www.netdoctor.co.uk/sex_relationships/facts/contraceptivecoil.htm | accessdate = 2006-07-05 }}</ref> | ||
==Hormonal | ==Hormonal Uterine Devices== | ||
{{main|IntraUterine System}} | {{main|IntraUterine System}} | ||
<!-- Deleted image removed: [[Image:IUDCPMirena.gif|thumb|150px|Photo of LNG IUS]] --> | <!-- Deleted image removed: [[Image:IUDCPMirena.gif|thumb|150px|Photo of LNG IUS]] --> | ||
| Line 168: | Line 169: | ||
A lower-dose T-shaped IntraUterine System named Femilis is being developed by Contrel, a Belgian company. Contrel also manufactures the FibroPlant-LNG, a frameless IUS. FibroPlant is anchored to the fundus of the uterus as the GyneFix IUD is. Although a number of trials have shown positive results, FibroPlant is not yet commercially available.<!-- | A lower-dose T-shaped IntraUterine System named Femilis is being developed by Contrel, a Belgian company. Contrel also manufactures the FibroPlant-LNG, a frameless IUS. FibroPlant is anchored to the fundus of the uterus as the GyneFix IUD is. Although a number of trials have shown positive results, FibroPlant is not yet commercially available.<!-- | ||
--><ref>{{cite journal | title = New Contraceptive Choices | journal = Population Reports, INFO Project, Center for Communication Programs | volume = M | issue = 19 | publisher = The Johns Hopkins School of Public Health | year = April 2005 | url = http://www.infoforhealth.org/pr/m19/ | accessdate = 2006-07-14 }} [http://www.infoforhealth.org/pr/m19/m19chap9.shtml Chapter 9: Intrauterine Devices].</ref> | --><ref>{{cite journal | title = New Contraceptive Choices | journal = Population Reports, INFO Project, Center for Communication Programs | volume = M | issue = 19 | publisher = The Johns Hopkins School of Public Health | year = April 2005 | url = http://www.infoforhealth.org/pr/m19/ | accessdate = 2006-07-14 }} [http://www.infoforhealth.org/pr/m19/m19chap9.shtml Chapter 9: Intrauterine Devices].</ref> | ||
==Related Chapters== | |||
== | |||
* [[Vas-occlusive contraception]] | * [[Vas-occlusive contraception]] | ||
Revision as of 19:47, 21 May 2013
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
An intra-uterine device (intra meaning within, and uterine meaning of the uterus) is a birth control device also known as an IUD or a coil (this colloquialism is based on the coil-shaped design of early IUDs). It is a device placed in the uterus and is the world's most widely used method of reversible birth control,[1] currently used by nearly 160 million women (just over two-thirds of whom are in China where it is the most widely used birth control method, surpassing sterilization).[2] The device has to be fitted inside or removed from the uterus by a doctor or qualified medical practitioner. It remains in place the entire time pregnancy is not desired. Depending on the type, a single IUD is approved for 5 to 10 years use (the copper T 380A is effective for at least 12 years).[3]
Types of IUDs
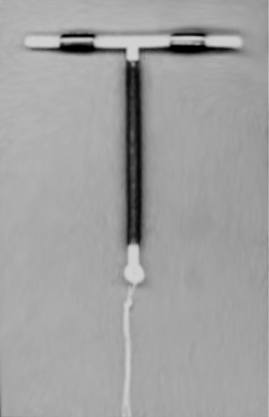
There are two broad categories of intrauterine contraceptive devices: inert and copper-based devices, and hormonally-based devices that work by releasing a progestogen.
In the United States, there are two types of intrauterine contraceptive available; the copper Paragard and the hormonal Mirena. In the U.S., both of these contraceptives are referred to as IUDs.[4]
In the United Kingdom, the term IUD only refers to inert or copper-containing devices; seven types of copper-containing IUDs are available in the UK. There, hormonal uterine contraceptives are considered a different form of contraception than copper IUDs, and they are distinguished with the term IntraUterine System or IUS.[5][6]
Most non-hormonal IUDs have a plastic T-shaped frame that is wound around with pure electrolytic copper wire and/or has copper collars (sleeves). Paragard's T 380a is 32 mm (1.26") in the horizontal direction (top of the T), and 36 mm (1.42") in the vertical direction (leg of the T). In some IUDs, such as the Nova T 380, the pure copper wire has a silver core which has been shown to prevent breaking of the wire.[5][7] The arms of the frame hold the IUD in place near the top of the uterus. The GyneFix does not have a T-shape, but rather is a loop that holds several copper tubes. The GyneFix is held in place by a suture to the fundus of the uterus. All copper-containing IUDs have a number as part of their name. This is the surface area of copper (in square millimeters) the IUD provides.
Effectiveness and Mechanism of Contraception
All second-generation copper-T IUDs have failure rates of less than 1% per year, and cumulative 10-year failure rates of 2-6%.[8] A large World Health Organization trial reported a cumulative 12-year failure rate of 2.2% for the T 380A (ParaGard) (an average failure rate of 0.18% per year over 12 years), equivalent to a cumulative 10-year failure rate of 1.8% following tubal ligation.[3] The frameless GyneFix also has a failure rate of less than 1% per year.[9] Worldwide, older IUD models with lower effectiveness rates are no longer produced.[10]
The presence of a device in the uterus prompts the release of leukocytes and prostaglandins by the endometrium. These substances are hostile to both sperm and eggs; the presence of copper increases this spermicidal effect.[11][12]
Some barrier contraceptives protect against STDs. Hormonal contraceptives reduce the risk of developing pelvic inflammatory disease (PID), a serious complication of certain STDs. IUDs, by contrast, do not protect against STDs or PID.[13]
Non-hormonal (copper) IUDs are considered safe to use while breastfeeding.[2]
While the primary mechanism of action of the IUD is spermicidal/ovicidal, post-fertilization mechanisms are believed by some Christian pro-life physicians to contribute significantly to their effectiveness.[14][15] Because many pro-life individuals and organizations define fertilization as the beginning of pregnancy, this possible secondary mechanism of action has led some pro-life individuals and organizations to label the IUD an abortifacient.
Contraindications
The WHO Medical Eligibility Criteria for Contraceptive Use and RCOG Faculty of Family Planning & Reproductive Health Care (FFPRHC) UK Medical Eligibility Criteria for Contraceptive Use list the following as conditions where insertion of a copper IUD is not usually recommended (category 3) or conditions where a copper IUD should not be inserted (category 4):[16][17]
Category 3
Conditions where the theoretical or proven risks usually outweigh the advantages of inserting a copper IUD:
- Postpartum between 48 hours and 4 weeks (increased IUD expulsion rate with delayed postpartum insertion)
- Benign gestational trophoblastic disease
- Ovarian cancer
- Very high individual likelihood of exposure to gonorrhea or chlamydial STIs
- AIDS (unless clinically well on anti-retroviral therapy)
Category 4
Conditions which represent an unacceptable health risk if a copper IUD is inserted:
- Pregnancy
- Postpartum puerperal sepsis
- Immediately post-septic abortion
- Before evaluation of unexplained vaginal bleeding suspected of being a serious condition
- Malignant gestational trophoblastic disease
- Cervical cancer (awaiting treatment)
- Endometrial cancer
- Distortions of the uterine cavity by uterine fibroids or anatomical abnormalities
- Current PID
- Current purulent cervicitis, chlamydial infection, or gonorrheal STIs
- Known pelvic tuberculosis
Some concern has been expressed that women with metal sensitivities to copper or nickel may experience adverse reactions from an IUD. The metal used in IUDs is 99.99% copper, with one study finding a maximum nickel content of 0.001%. Because nickel has a relatively high sensitizing potential, a few researchers suggested even this tiny amount might be problematic. A few case reports have attributed eczematous dermatitis and urticaria in a handful of users of copper-releasing IUDs to systemic copper or nickel allergic contact dermatitis. However, the daily metal absorption from an IUD is only a fraction of the normal daily absorption from food, and many dermatologists are skeptical that the symptoms described in the case reports were actually caused by metal sensitivity.[18][19][20]
While nulliparous women (women who have never given birth) are somewhat more likely to have side effects, this is not a contraindication for IUD use.
Some doctors prefer to insert the IUD during menstruation to verify that the woman is not pregnant at the time of insertion. However, IUDs may safely be inserted at any time during the menstrual cycle as long as it is reasonably certain the woman is not pregnant.[21] Insertion may be more comfortable if done midcycle, when the cervix is naturally dilated.[22]
Side Effects and Complications
Insertion of the IUD may introduce bacteria into the uterus. The insertion process carries a small, transient increased risk of pelvic inflammatory disease in the first 20 days following insertion.[13] It is very important that the provider use proper infection-prevention techniques during insertion.[23] Antibiotics should be given before insertion to women at high risk for endocarditis (inflammation of the membrane lining the heart), but should not be used routinely.[24]
During the placement appointment, the cervix is dilated in order to sound (measure) the uterus and insert the IUD. Cervix dilation is uncomfortable and, for some women, painful. Doctors often advise women to take painkillers before the procedure to reduce discomfort, and some will use a local anaesthetic.
After IUD insertion, menstrual periods are often heavier, more painful, or both - especially for the first few months after they are inserted. On average, menstrual blood loss increases by 20–50% after insertion of a copper-T IUD; increased menstrual discomfort is the most common medical reason for IUD removal.[25]
Complications include expulsion and uterine perforation. Uterine perforation is generally caused by an inexperienced provider and is very rare. Expulsion is more common in younger women, women who have not had children, and when an IUD is inserted immediately after childbirth or abortion. Women should check the string of the IUD at least once per menstrual cycle to verify that it is still in place.
The string(s) may be felt by some men during intercourse. If this is problematic, the provider may cut the strings even with the cervix, so they cannot be felt. Shortening the strings does prevent the woman from checking for expulsion, however.
The risk of ectopic pregnancy to a woman using an IUD is lower than the risk of ectopic pregnancy to a woman using no form of birth control. However, of pregnancies that do occur during IUD use, a higher than expected percentage (3–4%) are ectopic.[26]
Although the pregnancy rate during IUD use is very low (less than 1% per year), it is not a 100% effective method of birth control. If pregnancy does occur, presence of the IUD increases the risk of miscarriage, particularly during the second trimester. It also increases the risk of premature delivery. These increased risks end if the IUD is removed after pregnancy is discovered. Although the Dalkon Shield IUD was associated with septic abortions (infections associated with miscarriage), other brands of IUD are not. IUDs are also not associated with birth defects or other pregnancy complications.[27]
Use as Emergency Contraception
Intrauterine devices can be used as emergency contraception to prevent pregnancy up to 5 days after unprotected sexual intercourse, or sexual intercourse during which the primary contraception is believed to have failed (e.g. a condom was used, but it broke). Insertion of a copper-T IUD as emergency contraception is more than 99% effective, making it more effective than emergency contraceptive pills (ECP or 'morning-after pill').
Popularity
The IUD is the world's most widely used method of reversible birth control,[1] currently used by nearly 160 million women (just over two-thirds of whom are in China where it is the most widely used birth control method, surpassing sterilization).[2] Usage in many countries has been measured by surveys of married women of reproductive age. In this population, IUD use ranges from 5% in Belgium, to 18% in Scandinavia, 30% in Russia and China, and 40% in Kazakhstan.[28]
In the U.S., the ParaGard T 380A was approved by the FDA in 1984 and became available for use in 1988. It is still the only copper IUD approved for use in the U.S., and was used by 1.3% of women of reproductive age in a 2002 U.S. survey.[29] A wider variety of IUDs is available outside of the U.S. In the U.K., for example, seven brands are available: Flexi-T 300, Multi-Safe 375, Multi-Load Cu 375, Neo-Safe T380, Nova T 380, T-Safe 380A, and GyneFix - also called FlexiGard 330 or CuFix PP330.[5]
Hormonal Uterine Devices
Hormonal uterine devices do not increase bleeding as inert and copper-containing IUDs do. Rather, they reduce menstrual bleeding or prevent menstruation altogether, and can be used as a treatment for menorrhagia (heavy periods).
Although use of IntraUterine Systems results in much lower systemic progestogen levels than other very-low-dose progestogen-only hormonal contraceptives, they might possibly have some of the same side effects.
Progestasert was the first hormonal uterine device, developed in 1976[30] and manufactured until 2001.[31] It released progesterone, was replaced annually, and had a failure rate of 2% per year.[32]
As of 2007, the LNG-20 IUS - marketed as Mirena by Bayer - is the only IntraUterine System available. First introduced in 1990, it releases levonorgestrel (a progestogen) and may be used for five years.
A lower-dose T-shaped IntraUterine System named Femilis is being developed by Contrel, a Belgian company. Contrel also manufactures the FibroPlant-LNG, a frameless IUS. FibroPlant is anchored to the fundus of the uterus as the GyneFix IUD is. Although a number of trials have shown positive results, FibroPlant is not yet commercially available.[33]
Related Chapters
References
- ↑ 1.0 1.1 "What are the most widely used contraceptive methods across the world?". Births / Birth control. Institut national d'études démographiques (INED). 2006. Retrieved 2006-11-16.
- ↑ 2.0 2.1 World Health Organization (2002). "The intrauterine device (IUD)-worth singing about". Progress in Reproductive Health Research (60): 1–8.
- ↑ 3.0 3.1 World Health Organization (1997). "Long-term reversible contraception. Twelve years of experience with the TCu380A and TCu220C". Contraception. 56 (6): 341–52. PMID 9494767.
- ↑ Treiman K, Liskin L, Kols A, Rinehart W (1995). "IUDs—an update" (PDF). Popul Rep B (6): 1–35. PMID 8724322. Retrieved 2006-01-01.
- ↑ 5.0 5.1 5.2 "Contraceptive coils (IUDs)". NetDoctor.co.uk. 2006. Retrieved 2006-07-05.
- ↑ French, R (2004). "Hormonally impregnated intrauterine systems (IUSs) versus other forms of reversible contraceptives as effective methods of preventing pregnancy". Cochrane Database of Systematic Reviews (3). PMID 15266453. Unknown parameter
|coauthors=ignored (help) - ↑ Schering (2003). "Nova T380 Patient information leaflet (PIL)". Retrieved 2007-04-27. Unknown parameter
|month=ignored (help) - ↑ IUDs-An Update. Chapter 2.3: Effectiveness.
- ↑ O'Brien, PA (January 25, 2005). "Frameless versus classical intrauterine device for contraception". Cochrane Database of Systematic Reviews (1). PMID. Unknown parameter
|coauthors=ignored (help) - ↑ IUDs—An Update. Chapter 1: Background.
- ↑ "Mechanisms of the Contraceptive Action of Hormonal Methods and Intrauterine Devices (IUDs)". Family Health International. 2006. Retrieved 2006-07-05.
- ↑ Keller, Sarah (Winter 1996, Vol. 16, No. 2). "IUDs Block Fertilization". Network. Family Health International. Retrieved 2006-07-05. Check date values in:
|year=(help) - ↑ 13.0 13.1 Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O (1992). "Intrauterine devices and pelvic inflammatory disease: an international perspective". Lancet. 339 (8796): 785–8. PMID 1347812.
Grimes DA (2000). "Intrauterine device and upper-genital-tract infection". Lancet. 356 (9234): 1013–9. PMID 11041414.
Mishell Jr., Daniel R. (2004). "Contraception". In in Strauss III, Jerome F.; Barbieri, Robert L. (eds.). Yen and Jaffe's Reproductive Endocrinology (5th ed. ed.). Philadelphia: Elsevier Saunders. pp. pp. 899–938. ISBN 0-7216-9546-9.
Grimes, David A. (2004). "Intrauterine Devices (IUDs)". In in Hatcher, Robert A.; Trussell, James; Stewart, Felicia H.; Nelson, Anita L.; Cates Jr., Willard; Guest, Felicia; Kowal, Deborah (eds.). Contraceptive Technology (18th rev. ed. ed.). New York: Ardent Media. pp. pp. 495–530. ISBN 0-9664902-5-8.
Speroff, Leon; Darney, Philip D. (2005). "Intauterine Contraception: The IUD". A Clinical Guide for Contraception (4th ed. ed.). Philadelphia: Lippincott Williams & Wilkins. pp. pp. 221–257. ISBN 0-7817-6488-2.
Hall, Janet E. (2005). "Infertility and Fertility Control". In in Kasper Dennis L.; Braunwald, Eugene; Fauci, Anthony S.; Hauser, Stephen L.; Longo, Dan L.; Jameson, J. Larry (eds.). Harrison's Principles of Internal Medicine (16th ed. ed.). New York: McGraw-Hill. pp. pp. 279-83. ISBN 0-07-139140-1.
Soper, David E.; Mead, Philip B. (2005). "Infections of the Female Pelvis". In in Mandell, Gerald L.; Bennett, John E.; Dolin, Raphael (eds.). Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases (6th ed. ed.). Philadelphia: Elsevier Chuchill Livingston. pp. pp. 1372–81. ISBN 0-443-06643-4.
Glasier, Anna (2006). "Contraception". In in DeGroot, Leslie J.; Jameson, J. Larry (eds.). Endocrinology (5th ed. ed.). Philadelphia: Elsevier Saunders. pp. pp. 2993–3003. ISBN 0-7216-0376-9.Stubblefield, Phillip G.; Carr-Ellis, Sacheen; Kapp, Nathalie (2007). "Family Planning". In in Berek, Jonathan A. (ed.). Berek & Novak's Gynecology (14th ed. ed.). Philadelphia: Lippincott Williams & Wilkins. pp. pp. 247–311. ISBN 0-7817-6805-5. - ↑ Stanford J, Mikolajczyk R (2002). "Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects". Am J Obstet Gynecol. 187 (6): 1699–708. PMID 12501086.Smart Y, Fraser I, Clancy R, Roberts T, Cripps A (1982). "Early pregnancy factor as a monitor for fertilization in women wearing intrauterine devices". Fertil Steril. 37 (2): 201–4. PMID 6174375.
- ↑ Mikolajczyk, Rafael; Stanford, Joseph (2004). "Arguments against contraception--do they make sense to the general public? Importance of ethics, religion and "natural morality" in choice of family planning methods". In Fehring, Richard J,; Notare, Theresa (eds.). Integrating faith and science through natural family planning. Milwaukee: Marquette University Press. pp. pp. 229-32. ISBN 0874620112.
- ↑ WHO (2004). "Intrauterine devices (IUDs)". Medical Eligibility Criteria for Contraceptive Use (3rd ed. ed.). Geneva: Reproductive Health and Research, WHO. ISBN 92-4-156266-8.
- ↑ FFPRHC (2006). "The UK Medical Eligibility Criteria for Contraceptive Use (2005/2006)" (PDF). Retrieved 2007-01-11.
- ↑ Jouppila P, Niinimäki A, Mikkonen M (1979). "Copper allergy and copper IUD". Contraception. 19 (6): 631–7. PMID 487812.
- ↑ Frentz G, Teilum D (1980). "Cutaneous eruptions and intrauterine contraceptive copper device". Acta Derm Venereol. 60 (1): 69–71. PMID 6153839.
- ↑ Wohrl S, Hemmer W, Focke M, Gotz M, Jarisch R (2001). "Copper allergy revisited". J Am Acad Dermatol. 45 (6): 863–70. PMID 11712031.
- ↑ IUDs-An Update. Chapter 3: Insertion.
- ↑ "Understanding IUDs". Planned Parenthood Federation of America. July 2005. Retrieved 2006-07-22.
- ↑ IUDs—An Update. Sidebar: Infection Prevention for IUD Insertion and Removal.
- ↑ IUDs—An Update. Sidebar: Procedures for Providing IUDs.
- ↑ IUDs-An Update. Chapter 2.5: Bleeding and Pain.
- ↑ IUDs-An Update. Chapter 2.9:Ectopic Pregnancies.
- ↑ IUDs-An Update. Chapter 2.8: Intrauterine Pregnancy.
- ↑ IUDs—An Update. Worldwide Use - Developed Countries. Table 2: Worldwide Use of IUDs.
- ↑ Chandra, A (2005). "Fertility, Family Planning, and Reproductive Health of U.S. Women: Data From the 2002 National Survey of Family Growth" (PDF). Vital Health Stat. National Center for Health Statistics. 23 (25). Retrieved 2007-05-20. Unknown parameter
|coauthors=ignored (help) See Table 56. - ↑ IUDs—An Update. Chapter 2: Types of IUDs.
- ↑ Smith (pseudonym), Sydney (Saturday, March 8, 2003). "Contraceptive Concerns". medpundit: Commentary on medical news by a practicing physician. Retrieved 2006-07-16. Check date values in:
|date=(help) - ↑ "Birth Control Options: The Progestasert Intrauterine Device (IUD)". Wyoming Health Council. 2004. Retrieved 2006-07-16.
- ↑ "New Contraceptive Choices". Population Reports, INFO Project, Center for Communication Programs. The Johns Hopkins School of Public Health. M (19). April 2005. Retrieved 2006-07-14. Chapter 9: Intrauterine Devices.
de:Intrauterinpessar it:spirale (contraccettivo) he:התקן תוך רחמי lt:Gimdos spiralė nl:Spiraaltje no:Kobberspiral fi:Kierukka sv:Spiral (preventivmedel)
- Pages using duplicate arguments in template calls
- CS1 maint: Multiple names: authors list
- Pages with citations using unsupported parameters
- CS1 errors: dates
- CS1 maint: Multiple names: editors list
- CS1 maint: Extra text: editors list
- CS1 maint: Extra text
- Intrauterine contraception
- Obstetrics
- Gynecology
- Primary care