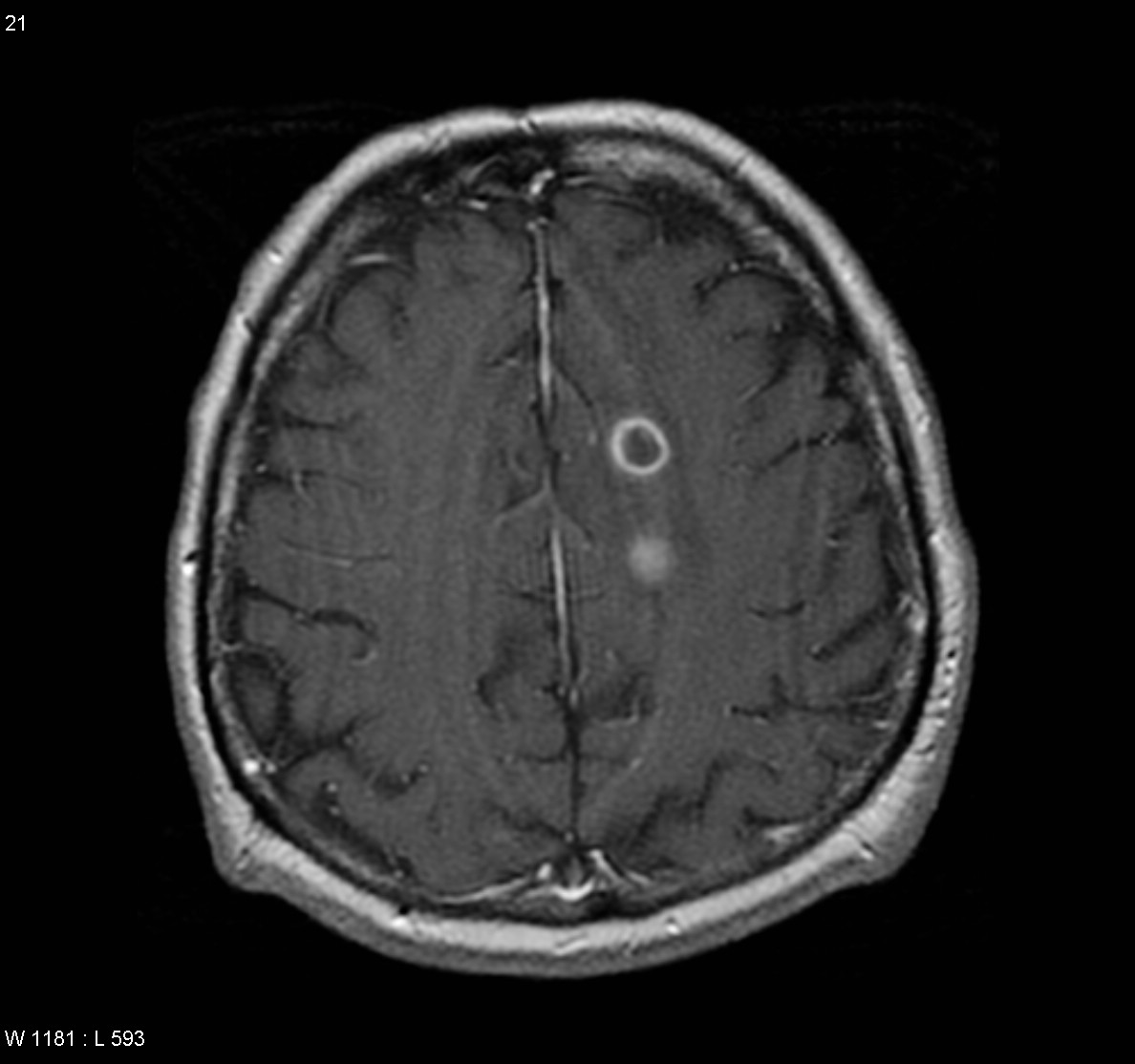
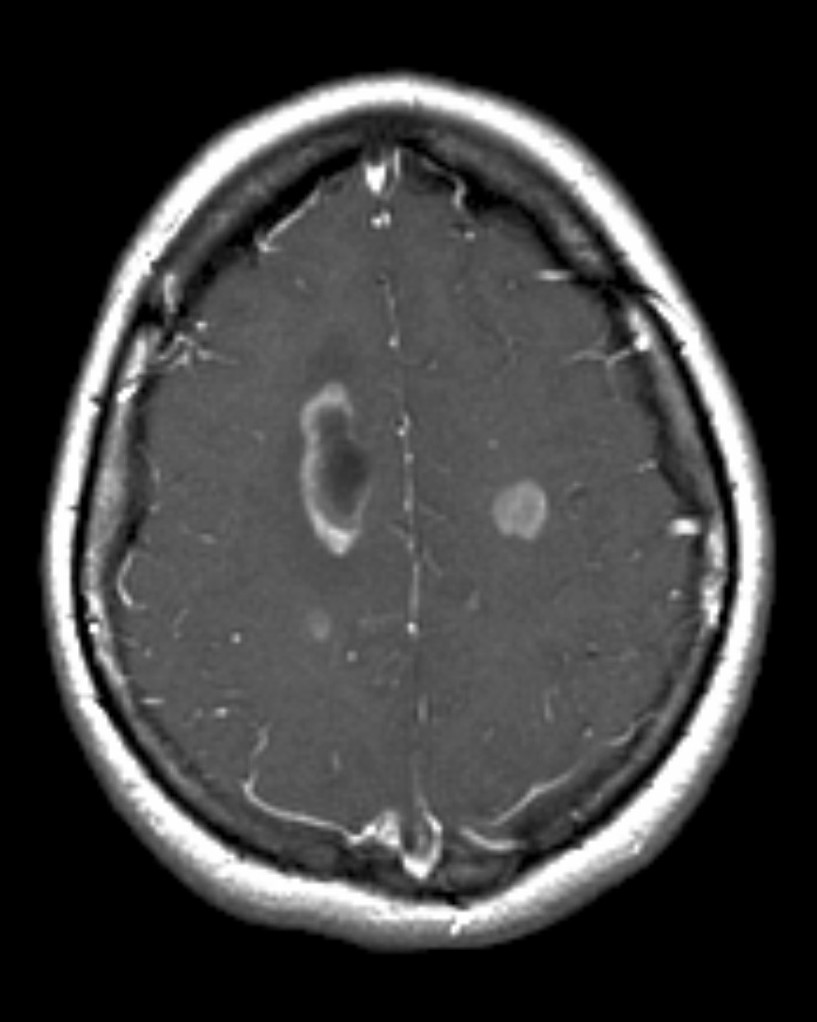
Intracerebral metastases
| Intracerebral metastases |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor in Editor: Cafer Zorkun, M.D., Ph.D. [2]
Intracerebral metastasis accounts for approximately 25-50% of intracranial tumors in hospitalized patients. The true incidence of brain metastasis is unknown, but recent estimates are as high as 200,000 cases per year in the United States alone. 80% of brain metastases can be accounted for by five primary tumor sites: lung, breast, skin (melanoma), kidney and the gastrointestinal tract. A population-based study of 169,444 cancer patients from 1973 to 2001 in Detroit revealed that overall, 10% of patients diagnosed with one of these five primaries went on to develop brain metastases. Specifically, 19.9% of lung cancers, 6.9% of melanomas, 6.5% of renal cancers, 5.1% of breast cancers and 1.8% of colorectal cancers metastasized to the brain.
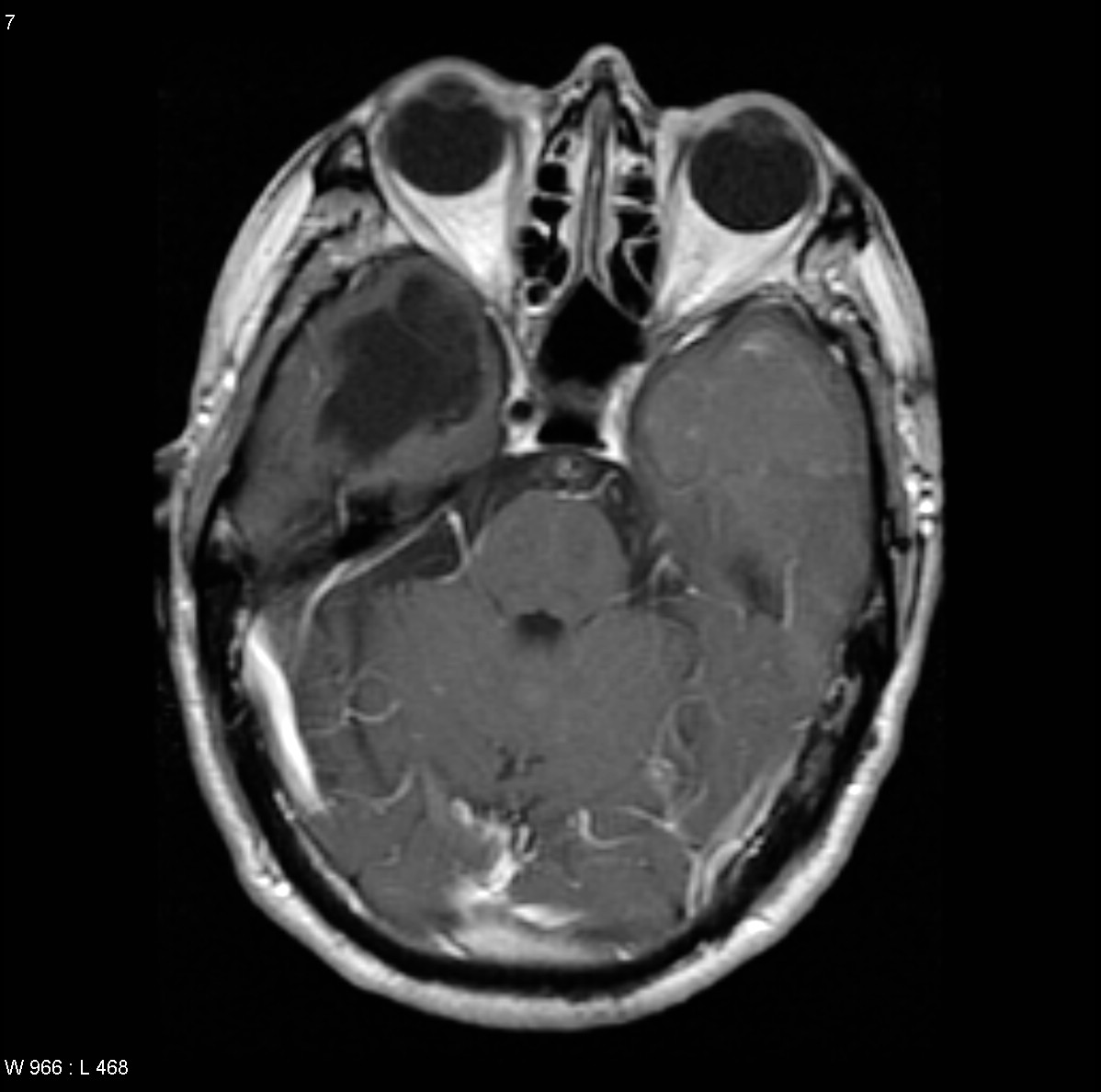
Parenchymal blood flow is an important determinant of the distribution of metastases. 80% of metastases localize to the cerebral hemispheres, 15% localize to the cerebellum and 3% localize to the basal ganglia. Often these tumors can be found at the gray/white matter junction.
Gross appearance
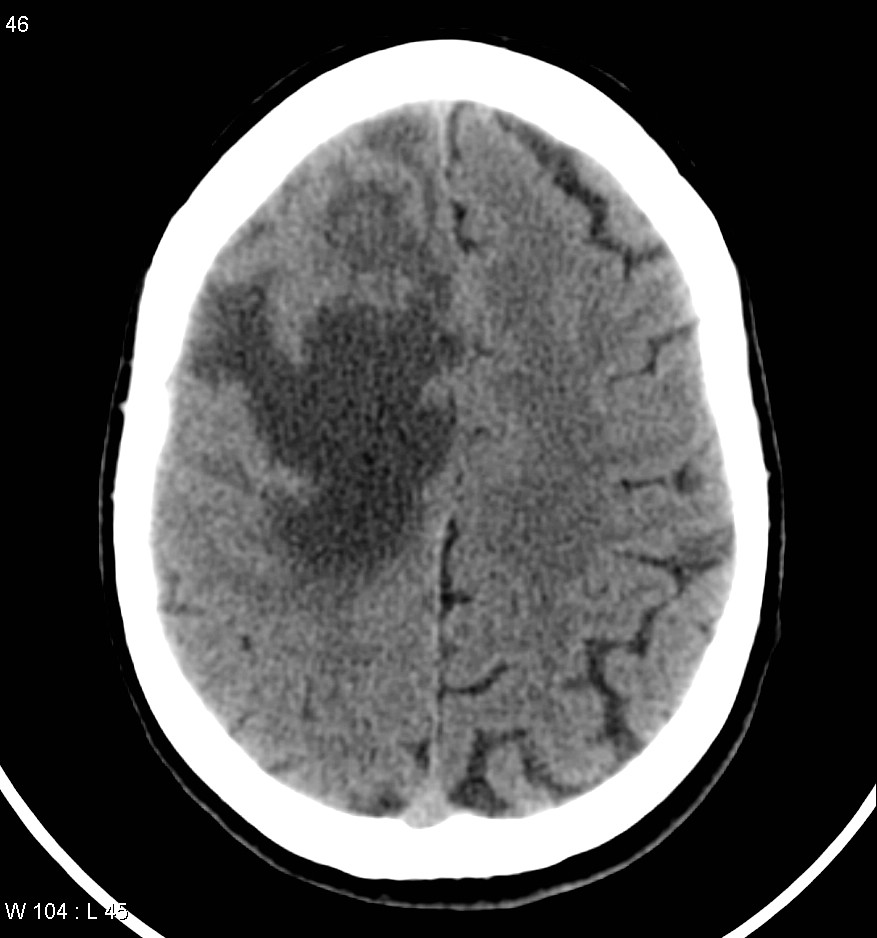
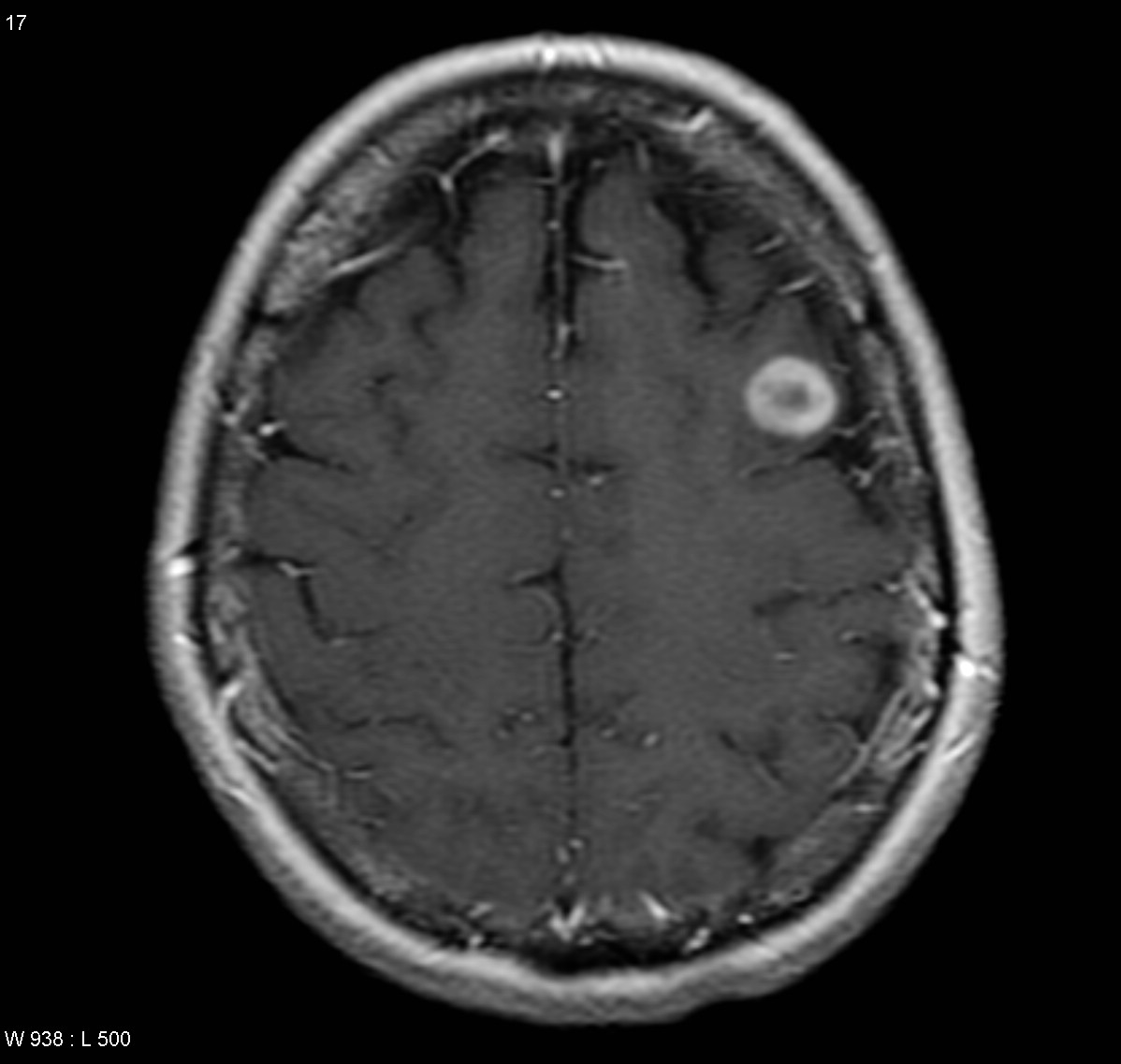
Typically metastases are sharply demarcated from the surrounding parenchyme and usually there is a zone of peritumoral edema out of proportion with the tumor size.
Microappearance
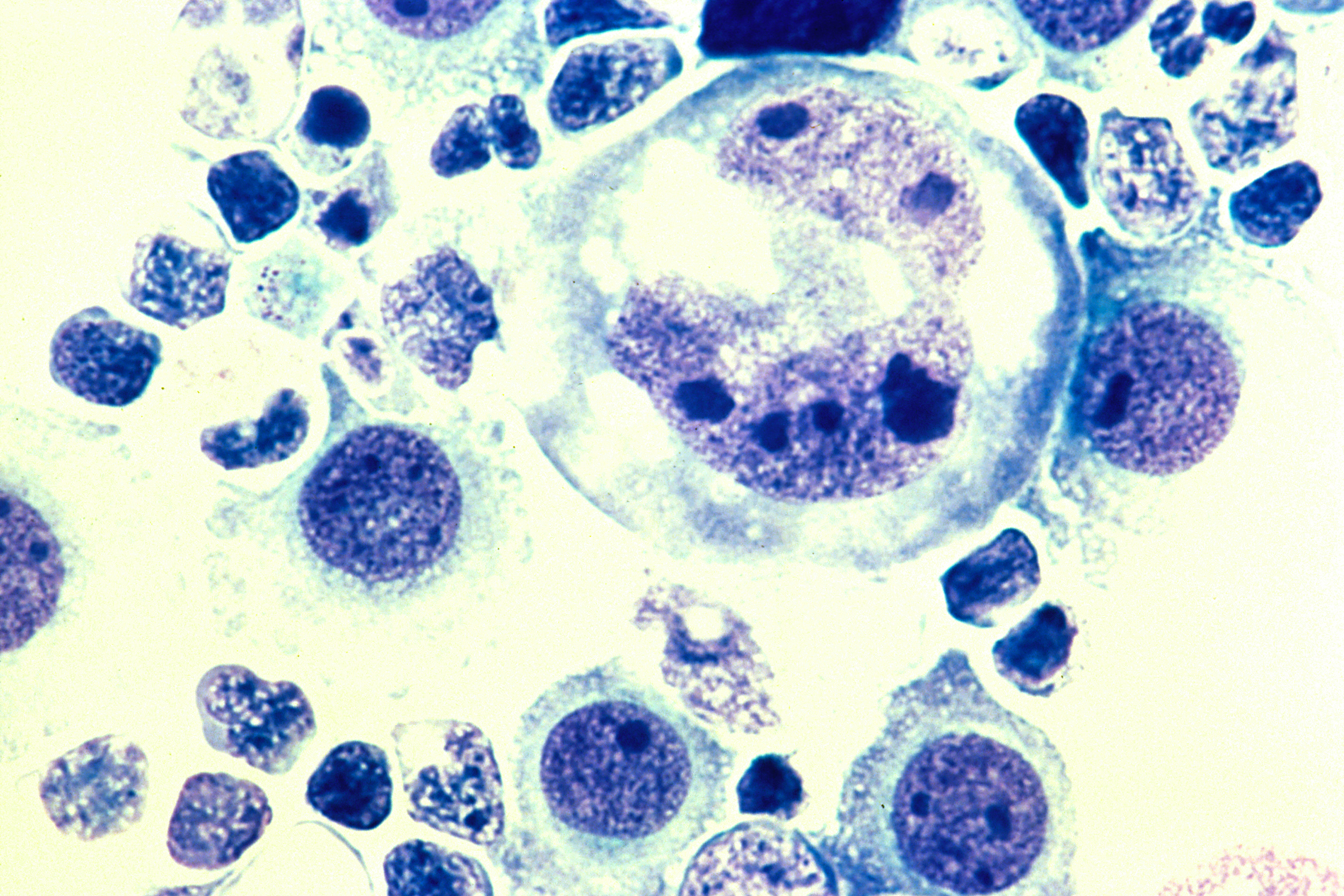
Typically well-demarcated with the exception of melanoma metastases.
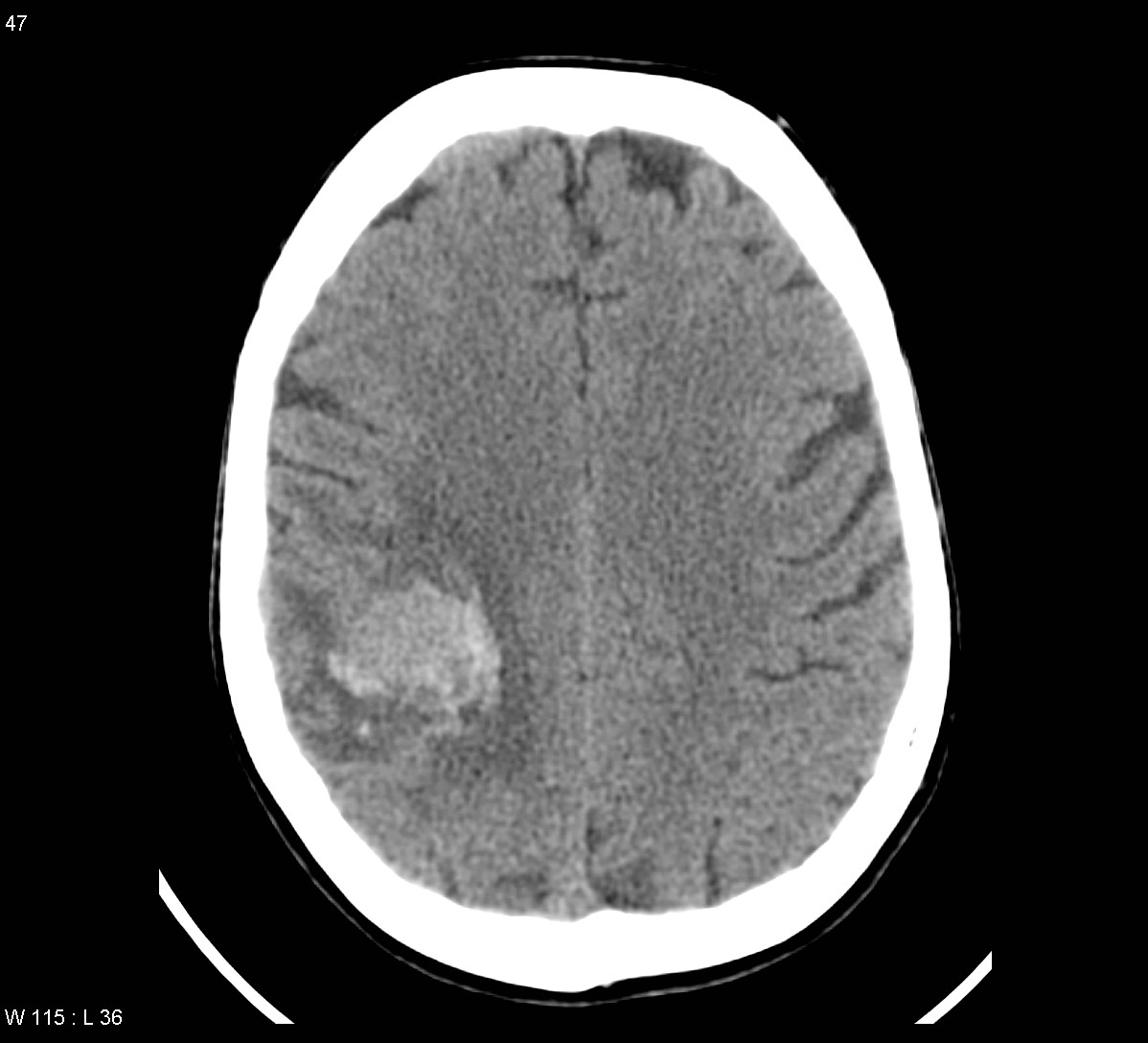
Radiographic findings
There is a great deal of variability in the appearance of these tumors, however some generalizations can be made.
CT
NECT: Iso to hypodense mass with anywhere from zero to marked peritumoral edema.
CECT: enhancement is also variable and can be intense, punctuate, nodular or ring-enhanced if the tumour has out grown it's blood supply.
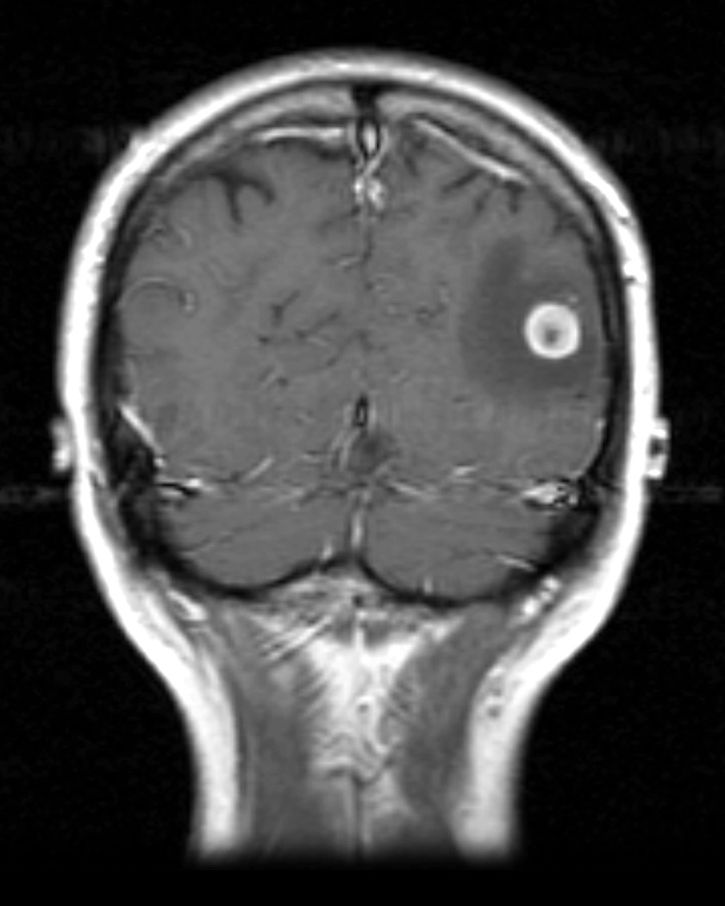
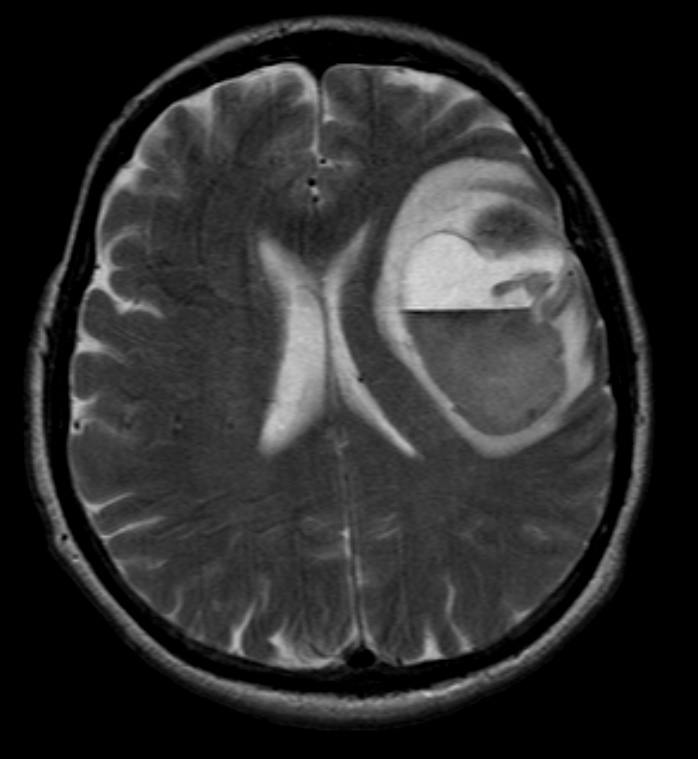
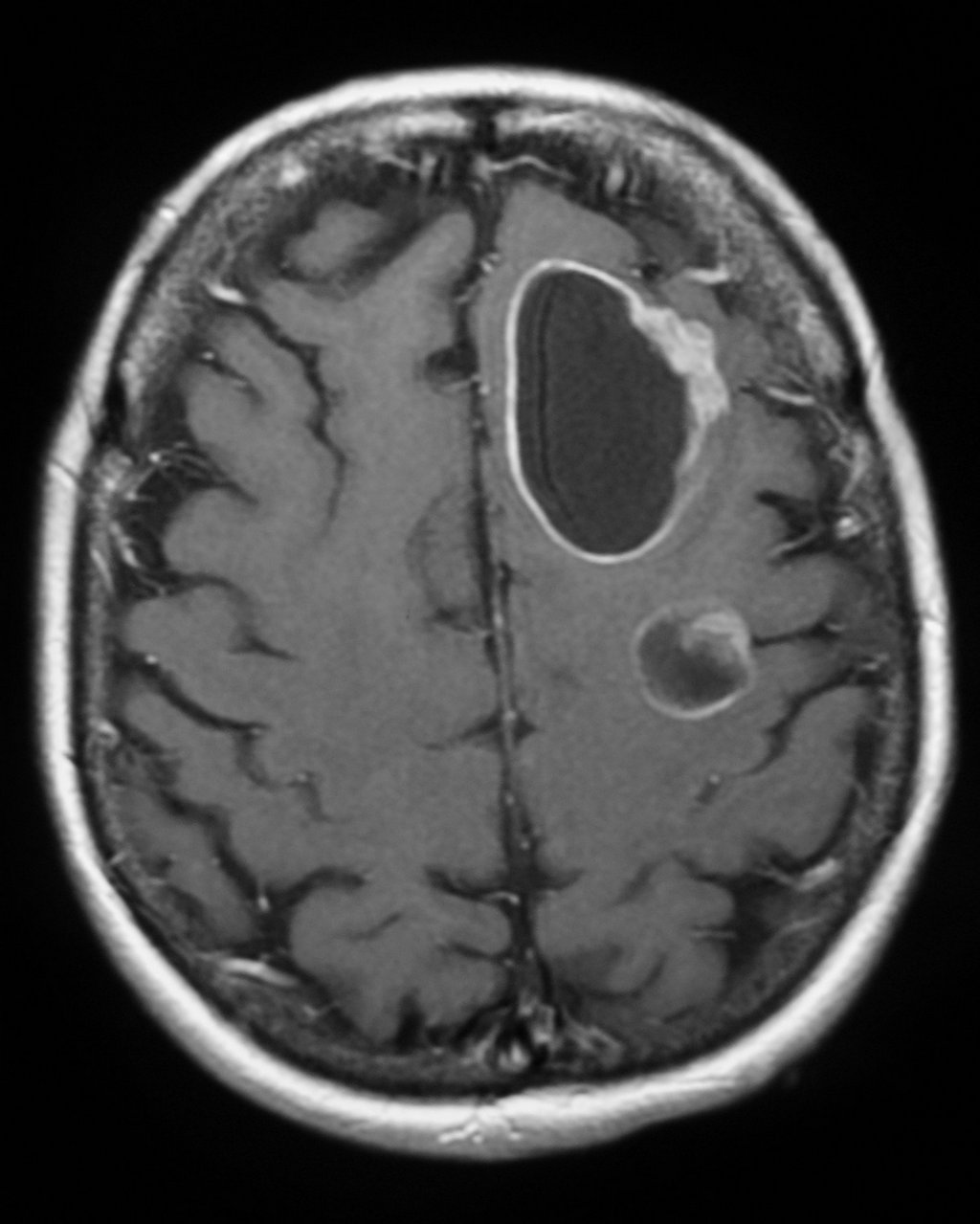
MRI
T1W: Typically iso to hypointense mass, however melanoma metastases are an exception to this rule (hyperintense due to the paramagnetic properties of melanin).
T2W: Typically hyperintense. If metastases are scattered the pattern may mimic vascular disease.
FLAIR: Typically hyperintense with hyperintense peritumoral edema.
T1 C+: The enhancement pattern can be uniform, punctuate, or ring-enhanced, but it is usually intense. Delayed sequences may show additional lesions, therefore contrast-enhance MR is the current standard for small met detection.
MRS: Intratumoral choline peak with no choline elevation in the peritumoral edema. Any tumor necrosis results in a lipid peak.
DWI: edema is out of proportion with tumour size and appears dark on trace-weighted DWI. Nuclear medicine
FDG PET
Generally considered the best imaging tool for metastases. However it can only detect metastases up to 1.5 cm in size, therefore CEMR is the gold standard to rule out small mets. Lung, breast, colorectal, head and neck, melanoma and thyroid mets are usually hypermetabolic. Mucinous adenocarcinoma and RCC are typically hypometabolic and gliomas and lymphomas are variable. Any central hypometabolism indicates necrosis.
Clinical presentation and prognosis
These patients commonly present with headache, seizure, mental status changes, ataxia, nausea and vomiting and visual disturbances. However, 10% of these patients may be asymptomatic.
Patients with brain mets have a mean survival of one month without treatment. With treatment, survival improves, but it is still dismal. The mean age of survival is still less than one year.
Differential Diagnosis
- Abscess: typically hypermetabolic with central hypometabolism signifying necrosis
- CVA
- Primary brain neoplasm
- Meningioma
- Post-treatment effects (post-surgical or post radiation): hypermetabolic acutely progressing to hypometabolic over time
- Epilepsy
Image Gallery for Differential Diagnosis
(Images shown below are courtesy of Radiopaedia.org)
-
Melanoma metastases
-
Bull's eye metastases
-
Melanoma metastases
-
Small cell lung ca metastases
-
Breast Ca Metastases
-
GBM (Glioblastoma multiforme)
-
Radiation necrosis
-
Lymphoma
-
Cerebral abscess
-
Demyelination
Treatment
Symptomatic: Corticosteroids are given to limit the effects of peritumoral edema. Hyperosmolar agents (eg mannitol) can be given to decrease ICP and anticonvulsants are given to prevent seizures. Recently, methylphenidate and donepezil have been used to improve cognition, mood and quality of life.
Therapeutic: Radiation (whole brain external beam or stereotactic for smaller masses), chemotherapy and surgical resection are done to prolong survival and palliate symptoms. Other than germ cell tumours, leukemias and lymphomas, palliation is the rule and curative therapy is the subject of case reports.
References
- Eichler AF, Loeffler JS. Multidisciplinary Management of Brain Metastases. The Oncologist 2007;12:884-898.
- Kumar V, Abbas AK, Fausto N. Robbins and Cotran Pathologic Basis of Disease. 7th ed. Philadelphia: Elsevier Saunders. 2005.
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan Detroit cancer surveillance system. J of Clin Oncol 2004;22(14):2865-72.
Source