Hematuria diagnostic evaluation
Steven C. Campbell, M.D., Ph.D.; Associate Editor(s)-in-Chief: Venkata Sivakrishna Kumar Pulivarthi M.B.B.S [1]
Overview
As the degree of hematuria increases, so does the likelihood of finding clinically significant lesions during evaluation. That is, the difference between the yield of life-threatening lesions in patients with gross versus microscopic hematuria has been found to be highly significant. Specifically, among patients with GH, 50% have been found to have a demonstrable cause, with 20% to 25% found to have a urologic malignancy, most commonly bladder cancer and kidney cancer. Given the increased frequency with which clinically significant findings are associated with GH, the recommended evaluation in this setting is relatively uniform. That is, patients presenting with GH in the absence of antecedent trauma or culture-documented UTI should be evaluated with a urine cytologic examination, cystoscopy, and upper tract imaging, preferably CT urogram. [1]
The initial evaluation of patients presenting with gross hematuria is 3-fold:[2]
- Assess hemodynamic stability
- Determine the underlying cause of hematuria (same for gross hematuria/ microscopic hematuria/ asymptomatic hematuria).[3]
- Ensure urinary drainage.
Diagnosis
Evaluation of patients with hematuria includes a focused history and physical examination, urinalysis and various blood tests. evaluation of hematuria is best performed in stepwise progression, beginning with simple and noninvasive investigations, followed by more aggressive interventions if positive results are obtained. Most importantly the lower urinary tract should be visualized using cystoscopy, usually using a flexible scope, and the upper tract imaged by a combination of modalities including plain X-ray, ultrasonography, intravenous urography or CT urography.
- Further, patients with GH must be assessed for hemodynamic stability with careful attention to vital signs, anemia with a complete blood count, and, for patients on anticoagulation, coagulation parameters to ensure that levels are within the therapeutic range. After initial stabilization, diagnostic evaluation should then proceed, with cause-specific management. [1]
Urine-based tests
A fresh sample of urine should be dipstick tested for proteinuria (renal disease) or nitrituria (infection). If abnormal the sample should be sent for microbiological assessment (microscopy and culture) and cytology.[4]
Dipstick test
A urine dipstick analysis is a highly sensitive measure for detection of blood, but it lacks specificity (sensitivity of 95% and a specificity of 75%). This translates into a large number of false positives, in which case, the urine dipstick is positive, but microscopy reveals fewer than 3RBC/HPF. This particular combination can be seen in the following benign or pathological circumstances:[5]
- Ingestion of certain foods: beets, blackberries, food coloring
- Ingestion of certain medications: Chloroquine, Ibuprofen, Iron, Sorbitol, Nitrofurantoin, Phenazopyridine, Urates or Rifampin (which often produces orange urine)
- Hemoglobinuria: often in the setting of hemolytic anemia
- Myoglobinuria: related to muscle damage (rhabdomyolysis), often after vigorous exercise or trauma
- Urinary tract infection: secondary to the action of bacterial peroxidases on the dipstick
- Delay in reading urine dipstick after submersion in urine
- presence of semen in urine.
Given the large number of situations in which a positive dipstick may not represent true hematuria, all urine samples that test positive on dipstick analysis must be sent for microscopy to confirm hematuria.
Microscopy and urine cytology
| Microscopy | Urine cytology |
|---|---|
| Microscopy is performed on urinary sediment (following centrifuging a fresh urine sample) and can quantify the number of erythrocytes.
Advantages
|
Urine cytology is the ‘gold standard’ urine-based test for detecting cancer.
Advantages
|
Positive Dipstick Test and Negitive Microscopic Results[6]
Patients who screen positive for hematuria with a urine dipstick test but have a negative follow-up microscopic examination should undergo three additional microscopic tests to rule out hematuria. If one of these repeat test results is positive on microscopic analysis, the patient is considered to have microscopic hematuria. If all three specimens are negative on microscopy, the patient does not require further evaluation for hematuria,6 and other causes of a positive dipstick test result, such as hemoglobinuria and myoglobinuria, should be considered.
Urine Culture
If a patient has microscopic hematuria in the presence of pyuria or bacteriuria, a urine culture should be obtained to rule out urinary tract infection. Culture-directed antibiotics should be administered, and a microscopic urinalysis should be repeated in six weeks to assess for resolution of the hematuria. If the hematuria has resolved after the infection has cleared, no further workup is needed. If hematuria persists, diagnostic evaluation should commence.[6]
Blood tests
- Complete blood count (to detect anemia)
- Coagulation studies (to detect hemoglobinopathies)
- Complement levels (to detect nephritic cause of hematuria)
- Serum urea, creatinine and electrolytes (to detect renal impairment)
Investigation of the lower urinary tract
Cytoscopy
It is a key component of the hematuria evaluation because it is the most reliable way to evaluate the bladder for the presence of bladder cancer and provides the opportunity to evaluate the urethra. Cystoscopy should be performed in all adults who meet criteria for hematuria evaluation who are 35 years of age or older and/or have risk factors for malignancy. Flexible cystoscope is useful for the inspection of the urethra and visualization of the bladder mucosa. It is quick, well tolerated and safe procedure. The detection of an abnormality will require subsequent rigid cystoscopy under anesthesia, whereby tissue can be obtained or treatment performed.[4] At the population level, bladder cancer is quite rare (<1 per 100,000) among persons 35 years old or younger, so cystoscopy may be omitted in persons younger than age 35 years without risk factors or clinical suspicion for bladder cancer or urethral pathology. The potential risks include discomfort, injury to the urethra, infection, and the need for additional procedures, such as biopsy.[3]
Investigation of the upper urinary tract
Evaluation of the upper urinary tract is more complex, and requires a balance between the low detection rate of pathology and the number or extent of tests required to visualize the urinary organs. No single imaging modality has the desired attributes of a high sensitivity and specificity, safety (low radiation exposure), low cost and applicability to lots of patients.[4] [6]
| Ultrasound (US) | Intravenous urography (IVU) | Computed tomography (CT) | Endoscopy/Fluoroscopy | MRI |
|---|---|---|---|---|
Advantages
Disadvantages
|
Advantages
Disadvantages
|
Advantages
Disadvantages
|
Advantages
Disadvantages
|
Advantages
Disadvantages
|
The gold standard investigation protocol for upper urinary tract would combine USS and IVU to evaluate the upper urinary tract. However, an IVU has a large radiation dosage with a small risk of reaction to contrast medium and detects upper tract UCC which are rare (less than 1% of all tumors presenting with hematuria). If looking for stone disease USS and a plain radiograph of the abdomen is recommended and IVU is reserved for equivocal cases, patients with persistent hematuria or patients with a high risk of UCC (elderly, smokers, occupational exposure).
Investigation of the glomerular cause of hematuria
- 24-hours urine collection should also be obtained to assess kidney function (e.g. Creatinine clearance/glomerular filtration rate, urine osmolality, sodium and albumin concentrations).
- Renal biopsy
Evaluation of patients with haematuria includes a focussed history and physical examination, urinalysis and various blood tests.Most importantly the lower urinary tract should be visualized using cystoscopy, usually using a flexible scope, and the upper tract imaged by a combination of modalities including plain X-ray, ultrasonography, intravenous urography or computed tomography urography.
The treatment options for haematuria depend on the underlying cause.
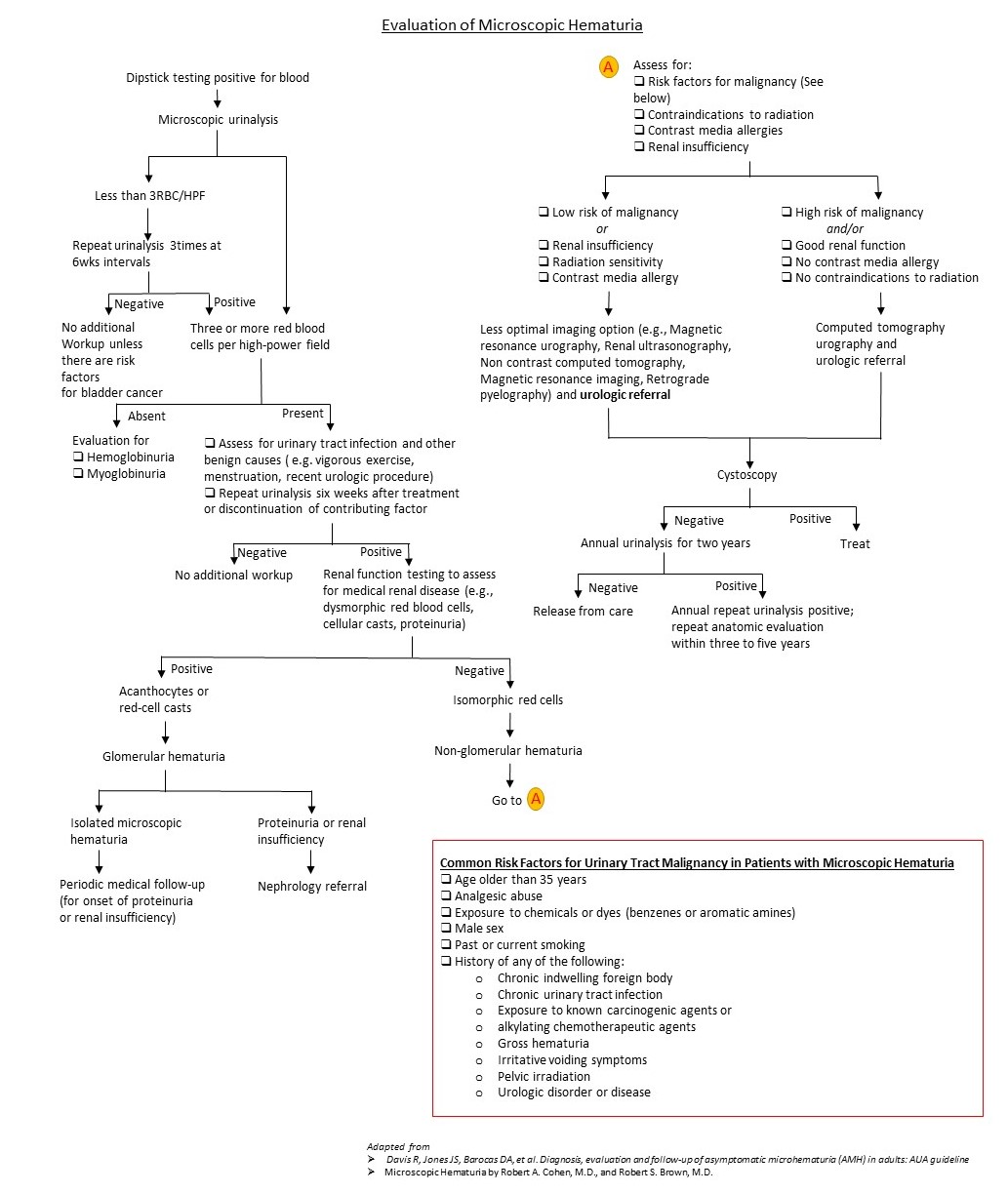
Algorithm for the Evaluation of Microscopic Hematuria [6][1]
Hematuria ' One-Stop ' Clinic
In recent years the ‘one-stop’ hematuria clinic has become popular for the investigation of hematuria as this enables synchronous urological and radiological evaluation of the patient, resulting in rapid diagnosis and treatment.This clinic has been set up to save you having repeated visits to the hospital. For most patients, in the course of one visit, all the tests needed to diagnose the cause of the blood in the urine will be undertaken. Occasionally some further tests will be necessary as a result of the findings at the first visit. In the morning you will have either an x-ray or a scan (KUB or Ultrasound) depending on the type of bleeding you have experienced. Early in the afternoon you will be seen by the consultant urologist or one of his team. You will then be examined and finally an internal inspection of your bladder will be performed. By the end of your visit you should know the outcome of all your tests and whether anything further needs to be done.[7]
References
- ↑ 1.0 1.1 1.2 Cohen, Robert A.; Brown, Robert S. (2003). "Microscopic Hematuria". New England Journal of Medicine. 348 (23): 2330–2338. doi:10.1056/NEJMcp012694. ISSN 0028-4793.
- ↑ Avellino GJ, Bose S, Wang DS (2016). "Diagnosis and Management of Hematuria". Surg Clin North Am. 96 (3): 503–15. doi:10.1016/j.suc.2016.02.007. PMID 27261791.
- ↑ 3.0 3.1 Pan, Cynthia G. (2006). "Evaluation of Gross Hematuria". Pediatric Clinics of North America. 53 (3): 401–412. doi:10.1016/j.pcl.2006.03.002. ISSN 0031-3955.
- ↑ 4.0 4.1 4.2 "www.surgeryjournal.co.uk".
- ↑ Amin, Nimisha; Zaritsky, Joshua J. (2011). "Hematuria": 258–261. doi:10.1016/B978-0-323-05405-8.00069-3.
- ↑ 6.0 6.1 6.2 6.3 Sharp VJ, Barnes KT, Erickson BA (2013) Assessment of asymptomatic microscopic hematuria in adults. Am Fam Physician 88 (11):747-54. PMID: 24364522
- ↑ Ooi WL, Lee F, Wallace DM, Hayne D (2011) 'One stop' haematuria clinic in Fremantle Hospital, Western Australia: a report of the first 500 patients. BJU Int 108 Suppl 2 ():62-6. DOI:10.1111/j.1464-410X.2011.10711.x PMID: 22085132