Enoxacin: Difference between revisions
(Blanked the page) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Drugbox | |||
| verifiedrevid = 461093764 | |||
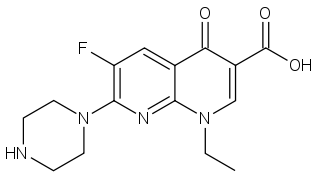
| IUPAC_name = 1-ethyl-6-fluoro-4-oxo-7-(piperazin-1-yl)-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid | |||
| image = Enoxacin.png | |||
| Drugs.com = {{drugs.com|monograph|oxistat}} | |||
| MedlinePlus = a601013 | |||
| routes_of_administration = Oral | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 74011-58-8 | |||
| ATC_prefix = J01 | |||
| ATC_suffix = MA04 | |||
| PubChem = 3229 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00467 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 3116 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 325OGW249P | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00310 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 157175 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 826 | |||
<!--Chemical data--> | |||
| C=15 | H=17 | F=1 | N=4 | O=3 | |||
| molecular_weight = 320.319 g/mol | |||
| smiles = Fc1c(nc2c(c1)C(=O)C(\C(=O)O)=C/N2CC)N3CCNCC3 | |||
| InChI = 1/C15H17FN4O3/c1-2-19-8-10(15(22)23)12(21)9-7-11(16)14(18-13(9)19)20-5-3-17-4-6-20/h7-8,17H,2-6H2,1H3,(H,22,23) | |||
| InChIKey = IDYZIJYBMGIQMJ-UHFFFAOYAG | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C15H17FN4O3/c1-2-19-8-10(15(22)23)12(21)9-7-11(16)14(18-13(9)19)20-5-3-17-4-6-20/h7-8,17H,2-6H2,1H3,(H,22,23) | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = IDYZIJYBMGIQMJ-UHFFFAOYSA-N | |||
}} | |||
__NOTOC__ | |||
{{SI}} | |||
{{CMG}} | |||
==Overview== | |||
'''Enoxacin'''<ref group=note>Enoxacin is sold under the following trade names: '''Almitil''', '''Bactidan''', '''Bactidron''', '''Comprecin''', '''Enoksetin''', '''Enoxen''', '''Enroxil''', '''Enoxin''', '''Enoxor''', '''Flumark''', '''Penetrex''', '''Gyramid''', '''Vinone'''.</ref> is an oral broad-spectrum [[fluoroquinolone]] [[antibacterial]] agent used in the treatment of [[urinary tract infection]]s and [[gonorrhea]]. [[Insomnia]] is a common adverse effect.<ref>{{Cite journal | last1 = Rafalsky | first1 = V. | last2 = Andreeva | first2 = I. | last3 = Rjabkova | first3 = E. | last4 = Rafalsky | first4 = Vladimir V | title = Quinolones for uncomplicated acute cystitis in women | journal = Cochrane Database Syst Rev | volume = 3 | issue = 3| pages = CD003597 | date= 2006 | doi = 10.1002/14651858.CD003597.pub2 | editor1-last = Rafalsky | editor1-first = Vladimir V | pmid=16856014}}</ref><ref>{{Cite journal | last1 = Mogabgab | first1 = WJ. | title = Recent developments in the treatment of sexually transmitted diseases | journal = Am J Med | volume = 91 | issue = 6A | pages = 140S–144S |date=Dec 1991 | doi = 10.1016/0002-9343(91)90327-T| pmid = 1767802 }}</ref> It is no longer available in the [[United States]]. | |||
It has been shown recently that it may have cancer inhibiting effect.<ref>http://www.pnas.org/content/early/2011/02/24/1014720108</ref> | |||
== Mechanism of action == | |||
Quinolones and fluoroquinolones are bactericidal drugs, eradicating bacteria by interfering with DNA replication. | |||
Like other fluoroquinolones, enoxacin functions by inhibiting bacterial [[DNA gyrase]] and [[topoisomerase IV]]. The inhibition of these enzymes prevents bacterial DNA replication, transcription, repair and recombination.<ref name="pmid8388200">{{cite journal |author=Yoshida H, Nakamura M, Bogaki M, Ito H, Kojima T, Hattori H, Nakamura S |title=Mechanism of action of quinolones against Escherichia coli DNA gyrase |journal=Antimicrob. Agents Chemother. |volume=37 |issue=4 |pages=839–45 | date=April 1993 |pmid=8388200 |pmc=187778 |doi= 10.1128/aac.37.4.839|url=http://aac.asm.org/cgi/pmidlookup?view=long&pmid=8388200 |accessdate=2014-09-24}}</ref><ref name="pmid3000292">{{cite journal |author=Wolfson JS, Hooper DC |title=The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro |journal=Antimicrob. Agents Chemother. |volume=28 |issue=4 |pages=581–6 | date=October 1985 |pmid=3000292 |pmc=180310 |doi= 10.1128/aac.28.4.581|url=http://aac.asm.org/cgi/pmidlookup?view=long&pmid=3000292 |accessdate=2014-09-24}}</ref> | |||
Enoxacin is active against many [[Gram-positive bacteria]].<ref group=note>Examples of Gram-positive bacteria include: [[Klebsiella pneumoniae]], [[Staphylococcus aureus]], [[Staphylococcus epidermidis]], [[Clostridium perfringens]].</ref> The quinolone is also active against [[Gram-negative bacteria]]<ref group=note>Gram-negative bacteria include: [[Acinetobacter]], [[Citrobacter]], [[Campylobacter]], [[Escherichia coli]], [[Haemophilus influenzae]], [[Moraxella catarrhalis]], [[Serratia marcescens]], [[Pseudomonas aeruginosa]], [[Proteus mirabilis]], [[Proteus vulgaris]], [[Salmonella]], [[Shigella flexneri]].</ref><ref name="pmid6229216">{{cite journal |author=Chin NX, Neu HC |title=In vitro activity of enoxacin, a quinolone carboxylic acid, compared with those of norfloxacin, new beta-lactams, aminoglycosides, and trimethoprim |journal=Antimicrob. Agents Chemother. |volume=24 |issue=5 |pages=754–63 | date=November 1983 |pmid=6229216 |pmc=185938 |doi= 10.1128/aac.24.5.754|url=http://aac.asm.org/cgi/pmidlookup?view=long&pmid=6229216 |accessdate=2014-09-24}}</ref><ref name="pmid6586712">{{cite journal |author=Wise R, Andrews JM, Danks G |title=In-vitro activity of enoxacin (CL-919), a new quinoline derivative, compared with that of other antimicrobial agents |journal=J. Antimicrob. Chemother. |volume=13 |issue=3 |pages=237–44 | date=March 1984 |pmid=6586712 |doi= 10.1093/jac/13.3.237|url=http://jac.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=6586712 |accessdate=2014-09-24}}</ref> | |||
== Pharmacokinetics == | |||
After oral administration enoxacin is rapidly and well absorbed from the gastrointestinal tract. The antibiotic is widely distributed throughout the body and in the different biological tissues. Tissue concentrations often exceed serum concentrations. The binding of enoxacin to serum proteins is 35 to 40%. | |||
The serum elimination half-life, in subjects with normal renal function, is approximately 6 hours. Approximately 60% of an orally administered dose is excreted in the urine as unchanged drug within 24 hours.<ref name="pmid6591851">{{cite journal |author=Wise R, Lockley R, Dent J, Webberly M |title=Pharmacokinetics and tissue penetration of enoxacin |journal=Antimicrob. Agents Chemother. |volume=26 |issue=1 |pages=17–9 | date=July 1984 |pmid=6591851 |pmc=179907 |doi= 10.1128/aac.26.1.17|url=http://aac.asm.org/cgi/pmidlookup?view=long&pmid=6591851 |accessdate=2014-09-24}}</ref><ref name="pmid3463542">{{cite journal |author=Wise R, Lister D, McNulty CA, Griggs D, Andrews JM |title=The comparative pharmacokinetics and tissue penetration of four quinolones including intravenously administered enoxacin |journal=Infection |volume=14 Suppl 3 |issue= |pages=S196–202 |year=1986 |pmid=3463542 |doi= 10.1007/bf01667843|url= |accessdate=2014-09-24}}</ref> | |||
A small amount of a dose of drug administered is excreted in the bile.<ref>Flowerdew, A., E. Walker, and S. J. Karran. "Evaluation of biliary pharmacokinetics of oral enoxacin, a new quinolone antibiotic." 14th International Congress of Chemotherapy, Kyoto. 1985.</ref> High concentrations of the fluoroquinolone are reached in the urinary tract and this fact ensures an antibacterial effect continued over time, particularly in this district. | |||
==Medical uses== | |||
Enoxacin can be used to treat a wide variety of infections, particularly [[gastroenteritis]] including infectious diarrhea, [[respiratory tract infections]], [[gonorrhea]]<ref name="pmid3111354">{{cite journal |author=van der Willigen AH, van der Hoek JC, Wagenvoort JH, van Vliet HJ, van Klingeren B, Schalla WO, Knapp JS, van Joost T, Michel MF, Stolz E |title=Comparative double-blind study of 200- and 400-mg enoxacin given orally in the treatment of acute uncomplicated urethral gonorrhea in males |journal=Antimicrob. Agents Chemother. |volume=31 |issue=4 |pages=535–8 | date=April 1987 |pmid=3111354 |pmc=174773 |doi= 10.1128/aac.31.4.535|url=http://aac.asm.org/cgi/pmidlookup?view=long&pmid=3111354 |accessdate=2014-09-24}}</ref> and [[urinary tract infections]].<ref name="pmid3162900">{{cite journal |author=Huttunen M, Kunnas K, Saloranta P |title=Enoxacin treatment of urinary tract infections in elderly patients |journal=J. Antimicrob. Chemother. |volume=21 Suppl B |issue= |pages=105–11 | date=February 1988 |pmid=3162900 |doi= 10.1093/jac/21.suppl_b.105|url=http://jac.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=3162900 |accessdate=2014-09-24}}</ref><ref name="pmid2764538">{{cite journal |author=Backhouse CI, Matthews JA |title=Single-dose enoxacin compared with 3-day treatment for urinary tract infection |journal=Antimicrob. Agents Chemother. |volume=33 |issue=6 |pages=877–80 | date=June 1989 |pmid=2764538 |pmc=284249 |doi= 10.1128/aac.33.6.877|url=http://aac.asm.org/cgi/pmidlookup?view=long&pmid=2764538 |accessdate=2014-09-24}}</ref> | |||
== Adverse effects == | |||
Enoxacin, like other fluoroquinolones, is known to trigger [[seizures]] or lower the [[seizure threshold]].<ref name="pmid8395790">{{cite journal |author=De Sarro A, Zappalá M, Chimirri A, Grasso S, De Sarro GB |title=Quinolones potentiate cefazolin-induced seizures in DBA/2 mice |journal=Antimicrob. Agents Chemother. |volume=37 |issue=7 |pages=1497–503 | date=July 1993 |pmid=8395790 |pmc=188001 |doi= 10.1128/aac.37.7.1497|url=http://aac.asm.org/cgi/pmidlookup?view=long&pmid=8395790 |accessdate=2014-09-25}}</ref> The compound should not be administered to patients with [[epilepsy]] or a personal history of previous convulsive attacks as may promote the onset of these disorders.<ref name="pmid2862357">{{cite journal |author=Simpson KJ, Brodie MJ |title=Convulsions related to enoxacin |journal=Lancet |volume=2 |issue=8447 |pages=161 | date=July 1985 |pmid=2862357 |doi= |url= |accessdate=2014-09-24}}</ref> | |||
== Contraindications == | |||
Enoxacin is contraindicated in subjects with a history of [[hypersensitivity]] to the substance or any other member of the quinolone class, or any component of the medicine. Enoxacin, like other fluoroquinolones, can cause degenerative changes in weightbearing joints of young animals. The compound should only be used in children | |||
when the expected benefits are outweigh the risks.<ref name="pmid12777590">{{cite journal |author=Chalumeau M, Tonnelier S, D'Athis P, Tréluyer JM, Gendrel D, Bréart G, Pons G |title=Fluoroquinolone safety in pediatric patients: a prospective, multicenter, comparative cohort study in France |journal=Pediatrics |volume=111 |issue=6 Pt 1 |pages=e714–9 | date=June 2003 |pmid=12777590 |doi= 10.1542/peds.111.6.e714|url=http://pediatrics.aappublications.org/cgi/pmidlookup?view=long&pmid=12777590 |accessdate=2014-09-24}}</ref><ref name="pmid16951028">{{cite journal |author= |title=The use of systemic fluoroquinolones |journal=Pediatrics |volume=118 |issue=3 |pages=1287–92 | date=September 2006 |pmid=16951028 |doi=10.1542/peds.2006-1722 |url=http://pediatrics.aappublications.org/cgi/pmidlookup?view=long&pmid=16951028 |accessdate=2014-09-24}}</ref> | |||
== Interactions == | |||
* [[Fenbufen]]: co-administration with some quinolones, including enoxacin may increase the risk of seizures. For this reason, concomitant administration of fenbufen and the quinolone should be avoided, as a precaution.<ref name="pmid3216153">{{cite journal |author=Morita H, Maemura K, Sakai Y, Kaneda Y |title=[A case of convulsion, loss of consciousness and subsequent acute renal failure caused by enoxacin and fenbufen] |language=Japanese |journal=Nippon Naika Gakkai Zasshi |volume=77 |issue=5 |pages=744–5 | date=May 1988 |pmid=3216153 |doi= 10.2169/naika.77.744|url= |accessdate=2014-09-25}}</ref><ref name="pmid1446880">{{cite journal |author=Hara Y, Ally A, Suzuki T, Murayama S |title=[Effects of drugs on the convulsions induced by the combination of a new quinolone antimicrobial, enoxacin, and a nonsteroidal anti-inflammatory drug, fenbufen, in mice] |language=Japanese |journal=Nippon Yakurigaku Zasshi |volume=100 |issue=4 |pages=301–5 | date=October 1992 |pmid=1446880 |doi= 10.1254/fpj.100.301|url= |accessdate=2014-09-25}}</ref><ref name="pmid9623721">{{cite journal |author=Masukawa T, Nakanishi K, Natsuki R |title=Role of nitric oxide in the convulsions following the coadministration of enoxacin with fenbufen in mice |journal=Jpn. J. Pharmacol. |volume=76 |issue=4 |pages=425–9 | date=April 1998 |pmid=9623721 |doi= 10.1254/jjp.76.425|url=http://joi.jlc.jst.go.jp/JST.JSTAGE/jjp/76.425?from=PubMed |accessdate=2014-09-25}}</ref><ref name="pmid9074952">{{cite journal |author=Masukawa T, Nakanishi K |title=Circadian variation in enoxacin-induced convulsions in mice coadministered with fenbufen |journal=Jpn. J. Pharmacol. |volume=73 |issue=2 |pages=175–7 | date=February 1997 |pmid=9074952 |doi= 10.1254/jjp.73.175|url=http://joi.jlc.jst.go.jp/JST.Journalarchive/jphs1951/73.175?from=PubMed |accessdate=2014-09-25}}</ref> | |||
* [[Theophylline]]: in patients treated concurrently with theophylline and enoxacin, concentrations of the [[methylxanthine]] in plasma arise due to a reduced metabolic clearance of theophylline.<ref name="pmid6145999">{{cite journal |author=Wijnands WJ, van Herwaarden CL, Vree TB |title=Enoxacin raises plasma theophylline concentrations |journal=Lancet |volume=2 |issue=8394 |pages=108–9 | date=July 1984 |pmid=6145999 |doi= |url= |accessdate=2014-09-25}}</ref><ref name="pmid3477409">{{cite journal |author=Niki Y, Soejima R, Kawane H, Sumi M, Umeki S |title=New synthetic quinolone antibacterial agents and serum concentration of theophylline |journal=Chest |volume=92 |issue=4 |pages=663–9 | date=October 1987 |pmid=3477409 |doi= 10.1378/chest.92.4.663|url=http://journal.publications.chestnet.org/article.aspx?volume=92&page=663 |accessdate=2014-09-25}}</ref><ref name="pmid8843297">{{cite journal |author=Mizuki Y, Fujiwara I, Yamaguchi T, Sekine Y |title=Structure-related inhibitory effect of antimicrobial enoxacin and derivatives on theophylline metabolism by rat liver microsomes |journal=Antimicrob. Agents Chemother. |volume=40 |issue=8 |pages=1875–80 | date=August 1996 |pmid=8843297 |pmc=163433 |doi= |url=http://aac.asm.org/cgi/pmidlookup?view=long&pmid=8843297 |accessdate=2014-09-25}}</ref><ref name="pmid3191935">{{cite journal |author=Sano M, Kawakatsu K, Ohkita C, Yamamoto I, Takeyama M, Yamashina H, Goto M |title=Effects of enoxacin, ofloxacin and norfloxacin on theophylline disposition in humans |journal=Eur. J. Clin. Pharmacol. |volume=35 |issue=2 |pages=161–5 |year=1988 |pmid=3191935 |doi= 10.1007/bf00609246|url= |accessdate=2014-09-25}}</ref> | |||
* [[Ranitidine]], [[sucralfate]], [[antacids]] containing [[magnesium]] or [[aluminum]], supplements containing [[calcium]], [[iron]], or [[zinc]]: co-administration with these substances can lead to therapeutic failure of the antibiotic due to decreased absorbment by the intestinal tract. For example magnesium or aluminum antacids turn enoxacin into insoluble salts that are not readily absorbed by the gastroenteric tract.<ref name="pmid2751276">{{cite journal |author=Grasela TH, Schentag JJ, Sedman AJ, Wilton JH, Thomas DJ, Schultz RW, Lebsack ME, Kinkel AW |title=Inhibition of enoxacin absorption by antacids or ranitidine |journal=Antimicrob. Agents Chemother. |volume=33 |issue=5 |pages=615–7 | date=May 1989 |pmid=2751276 |pmc=172500 |doi= 10.1128/aac.33.5.615|url=http://aac.asm.org/cgi/pmidlookup?view=long&pmid=2751276 |accessdate=2014-09-25}}</ref><ref name="pmid8494374">{{cite journal |author=Nix DE, Lebsack ME, Chapelsky M, Sedman AJ, Busch J, Norman A |title=Effect of oral antacids on disposition of intravenous enoxacin |journal=Antimicrob. Agents Chemother. |volume=37 |issue=4 |pages=775–7 | date=April 1993 |pmid=8494374 |pmc=187758 |doi= 10.1128/aac.37.4.775|url=http://aac.asm.org/cgi/pmidlookup?view=long&pmid=8494374 |accessdate=2014-09-25}}</ref><ref name="pmid8429114">{{cite journal |author=Misiak PM, Eldon MA, Toothaker RD, Sedman AJ |title=Effects of oral cimetidine or ranitidine on the pharmacokinetics of intravenous enoxacin |journal=J Clin Pharmacol |volume=33 |issue=1 |pages=53–6 | date=January 1993 |pmid=8429114 |doi= 10.1002/j.1552-4604.1993.tb03903.x|url=http://onlinelibrary.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=0091-2700&date=1993&volume=33&issue=1&spage=53 |accessdate=2014-09-25}}</ref> | |||
==References== | |||
{{Reflist|2}} | |||
{{QuinoloneAntiBiotics}} | |||
[[Category:Fluoroquinolone antibiotics]] | |||
[[Category:Withdrawn drugs]] | |||
[[Category:Naphthyridines]] | |||
[[Category:Piperazines]] | |||
[[Category:Drug]] | |||
Revision as of 15:01, 9 April 2015
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601013 |
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C15H17FN4O3 |
| Molar mass | 320.319 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
|
WikiDoc Resources for Enoxacin |
|
Articles |
|---|
|
Most recent articles on Enoxacin |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Enoxacin at Clinical Trials.gov Clinical Trials on Enoxacin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Enoxacin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Enoxacin Risk calculators and risk factors for Enoxacin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Enoxacin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Enoxacin[note 1] is an oral broad-spectrum fluoroquinolone antibacterial agent used in the treatment of urinary tract infections and gonorrhea. Insomnia is a common adverse effect.[1][2] It is no longer available in the United States.
It has been shown recently that it may have cancer inhibiting effect.[3]
Mechanism of action
Quinolones and fluoroquinolones are bactericidal drugs, eradicating bacteria by interfering with DNA replication. Like other fluoroquinolones, enoxacin functions by inhibiting bacterial DNA gyrase and topoisomerase IV. The inhibition of these enzymes prevents bacterial DNA replication, transcription, repair and recombination.[4][5] Enoxacin is active against many Gram-positive bacteria.[note 2] The quinolone is also active against Gram-negative bacteria[note 3][6][7]
Pharmacokinetics
After oral administration enoxacin is rapidly and well absorbed from the gastrointestinal tract. The antibiotic is widely distributed throughout the body and in the different biological tissues. Tissue concentrations often exceed serum concentrations. The binding of enoxacin to serum proteins is 35 to 40%. The serum elimination half-life, in subjects with normal renal function, is approximately 6 hours. Approximately 60% of an orally administered dose is excreted in the urine as unchanged drug within 24 hours.[8][9] A small amount of a dose of drug administered is excreted in the bile.[10] High concentrations of the fluoroquinolone are reached in the urinary tract and this fact ensures an antibacterial effect continued over time, particularly in this district.
Medical uses
Enoxacin can be used to treat a wide variety of infections, particularly gastroenteritis including infectious diarrhea, respiratory tract infections, gonorrhea[11] and urinary tract infections.[12][13]
Adverse effects
Enoxacin, like other fluoroquinolones, is known to trigger seizures or lower the seizure threshold.[14] The compound should not be administered to patients with epilepsy or a personal history of previous convulsive attacks as may promote the onset of these disorders.[15]
Contraindications
Enoxacin is contraindicated in subjects with a history of hypersensitivity to the substance or any other member of the quinolone class, or any component of the medicine. Enoxacin, like other fluoroquinolones, can cause degenerative changes in weightbearing joints of young animals. The compound should only be used in children when the expected benefits are outweigh the risks.[16][17]
Interactions
- Fenbufen: co-administration with some quinolones, including enoxacin may increase the risk of seizures. For this reason, concomitant administration of fenbufen and the quinolone should be avoided, as a precaution.[18][19][20][21]
- Theophylline: in patients treated concurrently with theophylline and enoxacin, concentrations of the methylxanthine in plasma arise due to a reduced metabolic clearance of theophylline.[22][23][24][25]
- Ranitidine, sucralfate, antacids containing magnesium or aluminum, supplements containing calcium, iron, or zinc: co-administration with these substances can lead to therapeutic failure of the antibiotic due to decreased absorbment by the intestinal tract. For example magnesium or aluminum antacids turn enoxacin into insoluble salts that are not readily absorbed by the gastroenteric tract.[26][27][28]
References
- ↑ Rafalsky, V.; Andreeva, I.; Rjabkova, E.; Rafalsky, Vladimir V (2006). Rafalsky, Vladimir V, ed. "Quinolones for uncomplicated acute cystitis in women". Cochrane Database Syst Rev. 3 (3): CD003597. doi:10.1002/14651858.CD003597.pub2. PMID 16856014.
- ↑ Mogabgab, WJ. (Dec 1991). "Recent developments in the treatment of sexually transmitted diseases". Am J Med. 91 (6A): 140S–144S. doi:10.1016/0002-9343(91)90327-T. PMID 1767802.
- ↑ http://www.pnas.org/content/early/2011/02/24/1014720108
- ↑ Yoshida H, Nakamura M, Bogaki M, Ito H, Kojima T, Hattori H, Nakamura S (April 1993). "Mechanism of action of quinolones against Escherichia coli DNA gyrase". Antimicrob. Agents Chemother. 37 (4): 839–45. doi:10.1128/aac.37.4.839. PMC 187778. PMID 8388200. Retrieved 2014-09-24.
- ↑ Wolfson JS, Hooper DC (October 1985). "The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro". Antimicrob. Agents Chemother. 28 (4): 581–6. doi:10.1128/aac.28.4.581. PMC 180310. PMID 3000292. Retrieved 2014-09-24.
- ↑ Chin NX, Neu HC (November 1983). "In vitro activity of enoxacin, a quinolone carboxylic acid, compared with those of norfloxacin, new beta-lactams, aminoglycosides, and trimethoprim". Antimicrob. Agents Chemother. 24 (5): 754–63. doi:10.1128/aac.24.5.754. PMC 185938. PMID 6229216. Retrieved 2014-09-24.
- ↑ Wise R, Andrews JM, Danks G (March 1984). "In-vitro activity of enoxacin (CL-919), a new quinoline derivative, compared with that of other antimicrobial agents". J. Antimicrob. Chemother. 13 (3): 237–44. doi:10.1093/jac/13.3.237. PMID 6586712. Retrieved 2014-09-24.
- ↑ Wise R, Lockley R, Dent J, Webberly M (July 1984). "Pharmacokinetics and tissue penetration of enoxacin". Antimicrob. Agents Chemother. 26 (1): 17–9. doi:10.1128/aac.26.1.17. PMC 179907. PMID 6591851. Retrieved 2014-09-24.
- ↑ Wise R, Lister D, McNulty CA, Griggs D, Andrews JM (1986). "The comparative pharmacokinetics and tissue penetration of four quinolones including intravenously administered enoxacin". Infection. 14 Suppl 3: S196–202. doi:10.1007/bf01667843. PMID 3463542.
|access-date=requires|url=(help) - ↑ Flowerdew, A., E. Walker, and S. J. Karran. "Evaluation of biliary pharmacokinetics of oral enoxacin, a new quinolone antibiotic." 14th International Congress of Chemotherapy, Kyoto. 1985.
- ↑ van der Willigen AH, van der Hoek JC, Wagenvoort JH, van Vliet HJ, van Klingeren B, Schalla WO, Knapp JS, van Joost T, Michel MF, Stolz E (April 1987). "Comparative double-blind study of 200- and 400-mg enoxacin given orally in the treatment of acute uncomplicated urethral gonorrhea in males". Antimicrob. Agents Chemother. 31 (4): 535–8. doi:10.1128/aac.31.4.535. PMC 174773. PMID 3111354. Retrieved 2014-09-24.
- ↑ Huttunen M, Kunnas K, Saloranta P (February 1988). "Enoxacin treatment of urinary tract infections in elderly patients". J. Antimicrob. Chemother. 21 Suppl B: 105–11. doi:10.1093/jac/21.suppl_b.105. PMID 3162900. Retrieved 2014-09-24.
- ↑ Backhouse CI, Matthews JA (June 1989). "Single-dose enoxacin compared with 3-day treatment for urinary tract infection". Antimicrob. Agents Chemother. 33 (6): 877–80. doi:10.1128/aac.33.6.877. PMC 284249. PMID 2764538. Retrieved 2014-09-24.
- ↑ De Sarro A, Zappalá M, Chimirri A, Grasso S, De Sarro GB (July 1993). "Quinolones potentiate cefazolin-induced seizures in DBA/2 mice". Antimicrob. Agents Chemother. 37 (7): 1497–503. doi:10.1128/aac.37.7.1497. PMC 188001. PMID 8395790. Retrieved 2014-09-25.
- ↑ Simpson KJ, Brodie MJ (July 1985). "Convulsions related to enoxacin". Lancet. 2 (8447): 161. PMID 2862357.
|access-date=requires|url=(help) - ↑ Chalumeau M, Tonnelier S, D'Athis P, Tréluyer JM, Gendrel D, Bréart G, Pons G (June 2003). "Fluoroquinolone safety in pediatric patients: a prospective, multicenter, comparative cohort study in France". Pediatrics. 111 (6 Pt 1): e714–9. doi:10.1542/peds.111.6.e714. PMID 12777590. Retrieved 2014-09-24.
- ↑ "The use of systemic fluoroquinolones". Pediatrics. 118 (3): 1287–92. September 2006. doi:10.1542/peds.2006-1722. PMID 16951028. Retrieved 2014-09-24.
- ↑ Morita H, Maemura K, Sakai Y, Kaneda Y (May 1988). "[A case of convulsion, loss of consciousness and subsequent acute renal failure caused by enoxacin and fenbufen]". Nippon Naika Gakkai Zasshi (in Japanese). 77 (5): 744–5. doi:10.2169/naika.77.744. PMID 3216153.
|access-date=requires|url=(help) - ↑ Hara Y, Ally A, Suzuki T, Murayama S (October 1992). "[Effects of drugs on the convulsions induced by the combination of a new quinolone antimicrobial, enoxacin, and a nonsteroidal anti-inflammatory drug, fenbufen, in mice]". Nippon Yakurigaku Zasshi (in Japanese). 100 (4): 301–5. doi:10.1254/fpj.100.301. PMID 1446880.
|access-date=requires|url=(help) - ↑ Masukawa T, Nakanishi K, Natsuki R (April 1998). "Role of nitric oxide in the convulsions following the coadministration of enoxacin with fenbufen in mice". Jpn. J. Pharmacol. 76 (4): 425–9. doi:10.1254/jjp.76.425. PMID 9623721. Retrieved 2014-09-25.
- ↑ Masukawa T, Nakanishi K (February 1997). "Circadian variation in enoxacin-induced convulsions in mice coadministered with fenbufen". Jpn. J. Pharmacol. 73 (2): 175–7. doi:10.1254/jjp.73.175. PMID 9074952. Retrieved 2014-09-25.
- ↑ Wijnands WJ, van Herwaarden CL, Vree TB (July 1984). "Enoxacin raises plasma theophylline concentrations". Lancet. 2 (8394): 108–9. PMID 6145999.
|access-date=requires|url=(help) - ↑ Niki Y, Soejima R, Kawane H, Sumi M, Umeki S (October 1987). "New synthetic quinolone antibacterial agents and serum concentration of theophylline". Chest. 92 (4): 663–9. doi:10.1378/chest.92.4.663. PMID 3477409. Retrieved 2014-09-25.
- ↑ Mizuki Y, Fujiwara I, Yamaguchi T, Sekine Y (August 1996). "Structure-related inhibitory effect of antimicrobial enoxacin and derivatives on theophylline metabolism by rat liver microsomes". Antimicrob. Agents Chemother. 40 (8): 1875–80. PMC 163433. PMID 8843297. Retrieved 2014-09-25.
- ↑ Sano M, Kawakatsu K, Ohkita C, Yamamoto I, Takeyama M, Yamashina H, Goto M (1988). "Effects of enoxacin, ofloxacin and norfloxacin on theophylline disposition in humans". Eur. J. Clin. Pharmacol. 35 (2): 161–5. doi:10.1007/bf00609246. PMID 3191935.
|access-date=requires|url=(help) - ↑ Grasela TH, Schentag JJ, Sedman AJ, Wilton JH, Thomas DJ, Schultz RW, Lebsack ME, Kinkel AW (May 1989). "Inhibition of enoxacin absorption by antacids or ranitidine". Antimicrob. Agents Chemother. 33 (5): 615–7. doi:10.1128/aac.33.5.615. PMC 172500. PMID 2751276. Retrieved 2014-09-25.
- ↑ Nix DE, Lebsack ME, Chapelsky M, Sedman AJ, Busch J, Norman A (April 1993). "Effect of oral antacids on disposition of intravenous enoxacin". Antimicrob. Agents Chemother. 37 (4): 775–7. doi:10.1128/aac.37.4.775. PMC 187758. PMID 8494374. Retrieved 2014-09-25.
- ↑ Misiak PM, Eldon MA, Toothaker RD, Sedman AJ (January 1993). "Effects of oral cimetidine or ranitidine on the pharmacokinetics of intravenous enoxacin". J Clin Pharmacol. 33 (1): 53–6. doi:10.1002/j.1552-4604.1993.tb03903.x. PMID 8429114. Retrieved 2014-09-25.
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Pages using citations with accessdate and no URL
- CS1 maint: Unrecognized language
- Template:drugs.com link with non-standard subpage
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Drugs with no legal status
- Fluoroquinolone antibiotics
- Withdrawn drugs
- Naphthyridines
- Piperazines
- Drug
- Pages with reference errors