Ebola causes
| style="background:#Template:Taxobox colour;"|Ebola virus | ||||||||
|---|---|---|---|---|---|---|---|---|
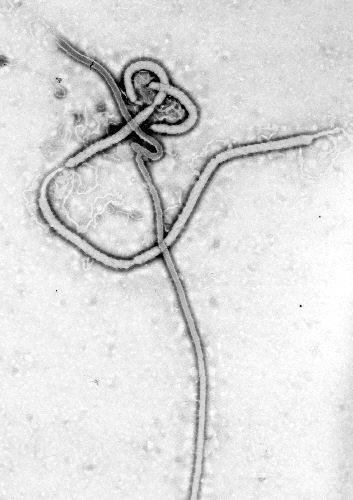 | ||||||||
| style="background:#Template:Taxobox colour;" | Virus classification | ||||||||
| ||||||||
| Type species | ||||||||
| Zaïre Ebolavirus | ||||||||
| Species | ||||||||
|
Reston Ebolavirus |
|
Ebola Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Postmortem Care |
|
Case Studies |
|
Ebola causes On the Web |
|
American Roentgen Ray Society Images of Ebola causes |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Ebola infection is caused by a virus that belongs to the family Filoviridae. Four viral subtypes have been reported to cause clinical illness in humans: Bundibugyo ebolavirus, Sudan ebolavirus, Tai Forest ebolavirus, and Zaire ebolavirus.
Taxonomy
Viruses; ssRNA viruses; ssRNA negative-strand viruses; Mononegavirales; Filoviridae; Ebolavirus[1]
- Ebolavirus
- Bundibugyo ebolavirus
- Reston ebolavirus
- Reston ebolavirus - Reston
- Reston ebolavirus - Reston (1989)
- Reston ebolavirus - Siena/Philippine-92
- Sudan ebolavirus
- Sudan ebolavirus - Boniface (1976)
- Sudan ebolavirus - Maleo (1979)
- Sudan ebolavirus - Nakisamata
- Sudan ebolavirus - Uganda (2000)
- Tai Forest ebolavirus
- Tai Forest virus - Côte d’Ivoire, Côte d’Ivoire, 1994
- Zaire ebolavirus
- Ebola virus - Mayinga, Zaire, 1976
- Zaire ebolavirus - Eckron (Zaire, 1976)
- Zaire ebolavirus - Gabon (1994-1997)
- Zaire ebolavirus - Zaire (1995)
- Unclassified Ebolavirus
- Ebola virus Yambio0401
- Ebola virus Yambio0402
- Ebola virus Yambio0403
- Ebola virus sp.
Virology
Structure
Electron micrographs of Ebola virus demonstrates the characteristic thread-like structure of a filovirus.[2] EBOV VP30 is around 288 amino acids long.[2] The virions are tubular and variable in shape and may resemble a "U", "6", coiled, circular, or branched shape. It is important to note that laboratory purification techniques, such as centrifugation, may contribute to the various shapes.[2] Virions are generally 80 nm in diameter.[2] They are variable in length, and can be up to 1400 nm long. On average, however, the length of a typical Ebola virus is closer to 1000 nm. In the center of the virion is a structure called nucleocapsid, which is formed of the helically wound viral genomic RNA complexed to the proteins NP, VP35, VP30 and L. The virus has an average diameter of 40-50 nm and contains a central channel of 20–30 nm in diameter. Virally encoded glycoprotein (GP) spikes 10 nm long and 10 nm apart are present on the outer viral envelope of the virion, which is derived from the host cell membrane. Between the envelope and the nucleocapsid exists a matrix space that contains the viral proteins VP40 and VP24.
Genome
Each virion contains one minor molecule of linear, single-stranded, negative-sense RNA, totaling 18959 to 18961 nucleotides in length. The 3′ terminus is not polyadenylated and the 5′ end is not capped. Only 472 nucleotides from the 3' end and 731 nucleotides from the 5' end are sufficient for replication.[2] The genome codes for seven structural proteins and one non-structural protein. The gene order is as follows: 3′ - leader - NP - VP35 - VP40 - GP/sGP - VP30 - VP24 - L - trailer - 5′. Leader and trailer regions are non-transcribed regions that carry important signals to control transcription, replication, and packaging of the viral genomes into new virions. The genomic material by itself is not infectious, because viral proteins such as RNA-dependent RNA polymerase are necessary for viral transcription and replication.
Life Cycle
- The virus attaches to host receptors through the GP (glycoprotein) surface peplomer and is endocytosed into vesicles in the host cell.
- Fusion of virus membrane with the vesicle membrane occurs; nucleocapsid is released into the cytoplasm.
- The encapsidated, negative-sense genomic ssRNA is used as a template for the synthesis (3'-5') of polyadenylated, monocistronic mRNAs using the viral RNA-dependent RNA polymerase.
- Translation of the mRNA into viral proteins occurs using the host cell's machinery.
- Post-translational processing of viral proteins occurs. GP0 (glycoprotein precursor) is cleaved to GP1 and GP2, which are heavily glycosylated. These two molecules assemble, first into heterodimers, and then into trimers to give the surface peplomers. SGP (secreted glycoprotein) precursor is cleaved to SGP and delta peptide, both of which are released from the cell.
- As viral protein levels rise, a switch occurs from translation to replication. Using the negative-sense genomic RNA as a template, a complementary positive-sense ssRNA is synthesized; this is then used as a template for the synthesis of new genomic negative-sense ssRNA, which is rapidly encapsidated.
- The newly-formed nucleocapsides and envelope proteins associate at the host cell's plasma membrane; budding occurs, and the virions are released.
Viral Reservoirs
Despite numerous studies, the wildlife reservoir of Ebolavirus has not yet been identified. Between 1976 and 1998, 30,000 mammals, birds, reptiles, amphibians, and arthropods were sampled from outbreak regions with no virus detected.[3] Ebolavirus has been detected in the carcasses of gorillas, chimpanzees, and duikers during outbreaks in 2001 and 2003 and these carcasses were deemed to be the source of initial human infection. However given the high mortality in these species, they are unlikely to be able to act as reservoirs.[3] Plants, arthropods, and birds have also been considered as reservoirs, however bats are considered the most likely candidate[4].
Bats
Bats were known to reside in the cotton factory in which the index cases for the 1976 and 1979 outbreaks were employed.[3] Of 24 plant species and 19 vertebrate species experimentally inoculated with Ebolavirus, only bats became infected.[5] The absence of clinical signs in these bats is characteristic of a reservoir species. In 2002-03, a survey of 1,030 animals from Gabon and the Republic of the Congo including 679 bats found Ebolavirus RNA in 13fruit bats (Hyspignathus monstrosus, Epomops franquetti and Myonycteris torquata).[6] Bats are also known to be the reservoirs for a number of related viruses including Nipah virus, Hendra virus andlyssaviruses.
Microscopic Pathology
The images below display key features of the Ebola virus.
-
This transmission electron micrograph (TEM) demonstrates the ultrastructural morphology displayed by an Ebola virus. Source: CDC microbiologist Frederick A. Murphy.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[7]
-
This transmission electron micrograph (TEM) demonstrates the ultrastructural morphologic changes in this tissue sample isolate.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[7]
-
Scanning electron micrograph (SEM) revealing ultrastructural morphologic features of the Ebola virus from the Ivory Coast of Africa.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[7]
-
Negatively-stained transmission electron micrograph (TEM) demonstrating the ultrastructural curvilinear morphologic features displayed by the Ebola virus from the Ivory Coast of Africa.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[7]
References
- ↑ "Taxonomy browser (Ebolavirus)".
- ↑ 2.0 2.1 2.2 2.3 2.4 Klenk, Hans-Dieter (2004). Ebola and Marburg Viruses, Molecular and Cellular Biology. Wymondham, Norfolk: Horizon Bioscience. ISBN 0954523237. Unknown parameter
|coauthors=ignored (help) - ↑ 3.0 3.1 3.2 Pourrut, Xavier (2005). "The natural history of Ebola virus in Africa". Microbes and Infection. 7 (7–8): 1005–1014. doi:10.1016/j.micinf.2005.04.006. Unknown parameter
|coauthors=ignored (help) - ↑ "Fruit bats may carry Ebola virus". BBC News. 2005-12-11. Retrieved 2008-02-25.
- ↑ Swanepoel, R (1996). "Experimental inoculation of plants and animals with Ebola virus". Emerging Infectious Diseases. 2: 321–325. Unknown parameter
|coauthors=ignored (help) - ↑ Leroy, Eric (2005). "Fruit bats as reservoirs of Ebola virus". Nature. 438: 575–576. doi:10.1038/438575a. Unknown parameter
|coauthors=ignored (help) - ↑ 7.0 7.1 7.2 7.3 "Public Health Image Library (PHIL), Centers for Disease Control and Prevention".
![This transmission electron micrograph (TEM) demonstrates the ultrastructural morphology displayed by an Ebola virus. Source: CDC microbiologist Frederick A. Murphy.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[7]](/images/9/9d/Ebola_virus2.png)
![This transmission electron micrograph (TEM) demonstrates the ultrastructural morphologic changes in this tissue sample isolate.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[7]](/images/7/78/Ebola_virus1.png)
![Scanning electron micrograph (SEM) revealing ultrastructural morphologic features of the Ebola virus from the Ivory Coast of Africa.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[7]](/images/c/c4/Ebola_virus3.png)
![Negatively-stained transmission electron micrograph (TEM) demonstrating the ultrastructural curvilinear morphologic features displayed by the Ebola virus from the Ivory Coast of Africa.Adapted from Public Health Image Library (PHIL), Centers for Disease Control and Prevention.[7]](/images/2/2e/Ebola_virus4.png)