Desirudin: Difference between revisions
No edit summary |
No edit summary |
||
| Line 134: | Line 134: | ||
* [[Renal Impairment]] | * [[Renal Impairment]] | ||
:* Iprivask must be used with caution in patients with [[renal impairment]], particularly in those with moderate and severe renal impairment ([[creatinine clearance]] ≤60 mL/min/1.73 m2 body surface area). Dose reductions by factors of three and nine are recommended for patients with moderate and severe [[renal impairment]] respectively. In addition, daily aPTT and serum creatinine monitoring are recommended for patients with moderate or severe renal impairment | :* Iprivask must be used with caution in patients with [[renal impairment]], particularly in those with moderate and severe renal impairment ([[creatinine clearance]] ≤60 mL/min/1.73 m2 body surface area). Dose reductions by factors of three and nine are recommended for patients with moderate and severe [[renal impairment]] respectively. In addition, daily [[aPTT]] and serum [[creatinine]] monitoring are recommended for patients with moderate or severe [[renal impairment]]. | ||
* Hemorrhagic Events | * Hemorrhagic Events | ||
:* Iprivask is not intended for intramuscular injection as local hematoma formation may result. | :* Iprivask is not intended for intramuscular injection as local [[hematoma]] formation may result. | ||
:* Iprivask, like other | :* Iprivask, like other [[anticoagulant]]s, should be used with caution in patients with increased risks of [[hemorrhage]] such as those with recent major surgery, organ biopsy or puncture of a non-compressible vessel within the last month; a history of [[hemorrhagic stroke]], intracranial or intraocular [[bleeding]] including diabetic (hemorrhagic) retinopathy; recent ischemic stroke, severe uncontrolled hypertension, bacterial endocarditis, a known hemostatic disorder (congenital or acquired, e.g. hemophilia, liver disease) or a history of gastrointestinal or pulmonary bleeding within the past 3 months. | ||
:* Bleeding can occur at any site during therapy with Iprivask. An unexplained fall in hematocrit or blood pressure should lead to a search for a bleeding site. | :* Bleeding can occur at any site during therapy with Iprivask. An unexplained fall in hematocrit or blood pressure should lead to a search for a bleeding site. | ||
Revision as of 03:04, 14 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
SPINAL/EPIDURAL HEMATOMAS:
|
Overview
Desirudin is a direct thrombin inhibitor that is FDA approved for the {{{indicationType}}} of deep vein thrombosis in patients undergoing elective hip replacement surgery. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hemorrhage, injection site mass, wound secretion, anemia, deep thrombophlebitis, and nausea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Prophylaxis of Deep Vein Thrombosis
- Dosing Information
- All patients should be evaluated for bleeding disorder risk before prophylactic administration of Iprivask.
- Initial Dosage
- In patients undergoing hip replacement surgery, the recommended dose of Iprivask is 15 mg every 12 hours administered by subcutaneous injection with the initial dose given up to 5 to 15 minutes prior to surgery, but after induction of regional block anesthesia, if used. Up to 12 days administration (average duration 9 to 12 days) of Iprivask has been well tolerated in controlled clinical trials.
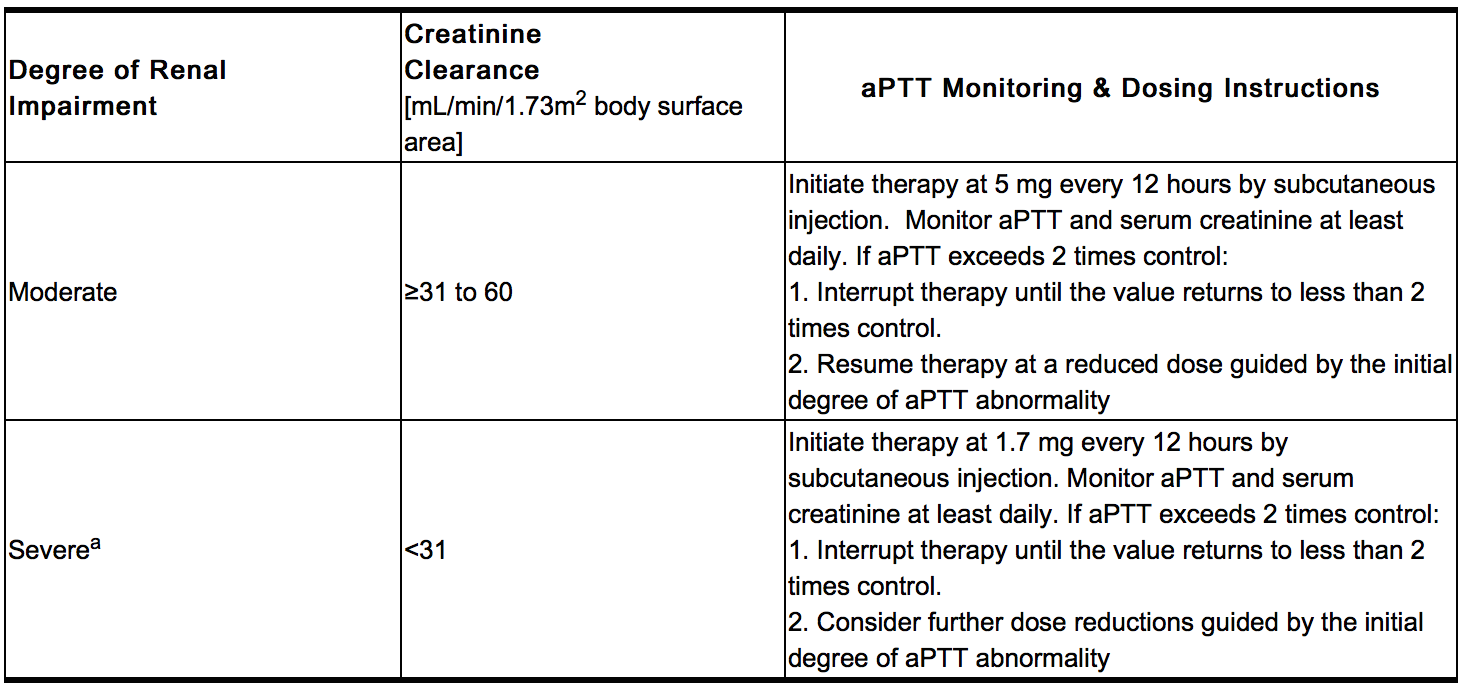
- Dose in Renal Impairment
- Dose in Hepatic Impairment
- In the absence of clinical studies in this population, dosing recommendations cannot be made at this time.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Desirudin in adult patients.
Non–Guideline-Supported Use
Prophylaxis of Coronary Thrombolysis in Acute Coronary Syndrome
- Equivalent or slightly better efficacy compared to heparin for thrombolysis in patients with acute coronary syndrome; desirudin administration is also associated with a lower early reinfarction rate.[1][2][3][4]
Percutaneous Transluminal Coronary Angioplasty
- Intravenous desirudin (20 mgbolus followed by an infusion of 0.16 mg/kg/hr for 24 hours) is associated with a reduced early cardiac events in patients undergoing percutaneous transluminal coronary angioplasty.[5][6]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Desirudin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Desirudin in pediatric patients.
Contraindications
- Iprivask is contraindicated in patients with known hypersensitivity to natural or recombinant hirudins, and in patients with active bleeding and/or irreversible coagulation disorders.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
SPINAL/EPIDURAL HEMATOMAS:
|
- Spinal/Epidural Anesthesia
- As with other anticoagulants, there is a risk of neuraxial hematoma formation with the concurrent use of desirudin and spinal/epidural anesthesia, which has the potential to result in long term or permanent paralysis. The risk may be greater with the use of post-operative indwelling catheters or the concomitant use of additional drugs affecting hemostasis such as NSAIDs (Non-Steroidal Anti-Inflammatory Drugs), platelet inhibitors or other anticoagulants. The risk may also be increased by traumatic or repeated neuraxial puncture.
- To reduce the potential risk of bleeding associated with the concurrent use of desirudin and epidural or spinal anesthesia/analgesia, the pharmacokinetic profile of the drug should be considered when scheduling or using epidural or spinal anesthesia in proximity to desirudin administration. The physician should consider placement of the catheter prior to initiating desirudin and removal of the catheter when the anticoagulant effect of desirudin is low.
- Should the physician decide to administer anticoagulation in the context of epidural/spinal anesthesia, extreme vigilance and frequent monitoring must be exercised to detect any signs and symptoms of neurological impairment such as midline back pain, sensory and motor deficits (numbness or weakness in lower limbs), bowel and/or bladder dysfunction. Patients should be instructed to inform their physician immediately if they experience any of the above signs or symptoms. If signs or symptoms of spinal hematoma are suspected, urgent diagnosis and treatment including spinal cord decompression should be initiated.
- The physician should consider the potential benefit versus risk before neuraxial intervention in patients anticoagulated or to be anticoagulated for thromboprophylaxis.
- Iprivask cannot be used interchangeably with other hirudins as they differ in manufacturing process and specific biological activity (ATUs). Each of these medicines has its own instructions for use.
- Iprivask must be used with caution in patients with renal impairment, particularly in those with moderate and severe renal impairment (creatinine clearance ≤60 mL/min/1.73 m2 body surface area). Dose reductions by factors of three and nine are recommended for patients with moderate and severe renal impairment respectively. In addition, daily aPTT and serum creatinine monitoring are recommended for patients with moderate or severe renal impairment.
- Hemorrhagic Events
- Iprivask is not intended for intramuscular injection as local hematoma formation may result.
- Iprivask, like other anticoagulants, should be used with caution in patients with increased risks of hemorrhage such as those with recent major surgery, organ biopsy or puncture of a non-compressible vessel within the last month; a history of hemorrhagic stroke, intracranial or intraocular bleeding including diabetic (hemorrhagic) retinopathy; recent ischemic stroke, severe uncontrolled hypertension, bacterial endocarditis, a known hemostatic disorder (congenital or acquired, e.g. hemophilia, liver disease) or a history of gastrointestinal or pulmonary bleeding within the past 3 months.
- Bleeding can occur at any site during therapy with Iprivask. An unexplained fall in hematocrit or blood pressure should lead to a search for a bleeding site.
- Antibodies/Re-exposure
- Antibodies have been reported in patients treated with hirudins. Potential for cross-sensitivity to hirudin products cannot be excluded. Irritative skin reactions were observed in 9/322 volunteers exposed to Iprivask by subcutaneous injection or IV bolus or infusion in single or multiple administrations of the drug. Allergic events were reported in <2% of patients who were administered desirudin in Phase III clinical trials. Allergic events were reported in 1% of patients receiving unfractionated heparin and 1% of patients receiving enoxaparin. Hirudin-specific IgE evaluations may not be indicative of sensitivity to Iprivask as this test was not always positive in the presence of symptoms. Very rarely, anti-hirudin antibodies have been detected upon re-exposure to desirudin. [See ADVERSE REACTIONS, Non-hemorrhagic Events, Allergic Reactions (6.1)]. Fatal anaphylactoid reactions have been reported during hirudin therapy.
- Hepatic Impairment/Liver Injury
- No information is available about the use of desirudin in patients with hepatic impairment/liver injury. Although Iprivask is not significantly metabolized by the liver, hepatic impairment or serious liver injury (e.g., liver cirrhosis) may alter the anticoagulant effect of Iprivask due to coagulation defects secondary to reduced generation of vitamin K-dependent coagulation factors. Iprivask should be used with caution in these patients.
- Laboratory Tests
- Activated partial thromboplastin time (aPTT) should be monitored daily in patients with increased risk of bleeding and/or renal impairment. Serum creatinine should be monitored daily in patients with renal impairment. Peak aPTT should not exceed two times control. Should peak aPTT exceed this level, dose reduction is advised based on the degree of aPTT abnormality [see DOSAGE and ADMINISTRATION, Recommended Dose (2.1)]. If necessary, therapy with desirudin should be interrupted until aPTT falls to less than two times control, at which time treatment with desirudin can be resumed at a reduced dose [(See DRUG INTERACTIONS (7) for information on use of Iprivask in conjunction with other drugs affecting coagulation]. Thrombin time (TT) is not a suitable test for routine monitoring of Iprivask therapy [see CLINICAL PHARMACOLOGY, Mechanism of Action (12.1)]. Dose adjustments based on serum creatinine may be necessary [see DOSAGE AND ADMINISTRATION, Dose in Renal Impairment (2.2)].
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Desirudin in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Desirudin in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Desirudin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Desirudin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Desirudin with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Desirudin with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Desirudin with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Desirudin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Desirudin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Desirudin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Desirudin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Desirudin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Desirudin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Desirudin in the drug label.
Condition1
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Desirudin in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Desirudin in the drug label.
Pharmacology
There is limited information regarding Desirudin Pharmacology in the drug label.
Mechanism of Action
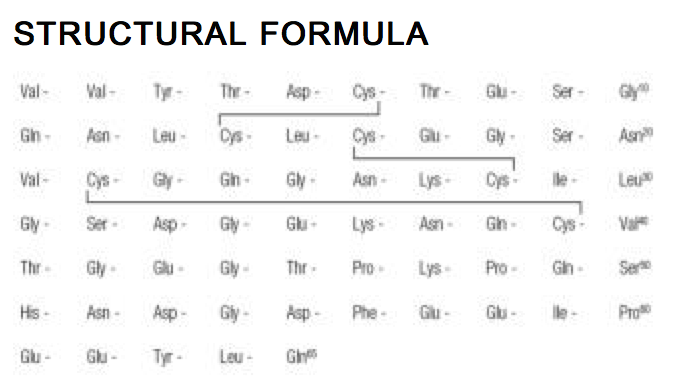
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Desirudin in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Desirudin in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Desirudin in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Desirudin in the drug label.
Condition1
- Description
How Supplied
Storage
There is limited information regarding Desirudin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Desirudin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Desirudin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Desirudin in the drug label.
Precautions with Alcohol
- Alcohol-Desirudin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Iprivask®[7]
Look-Alike Drug Names
- N/A[8]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "A comparison of recombinant hirudin with heparin for the treatment of acute coronary syndromes. The Global Use of Strategies to Open Occluded Coronary Arteries (GUSTO) IIb investigators". N Engl J Med. 335 (11): 775–82. 1996. doi:10.1056/NEJM199609123351103. PMID 8778585.
- ↑ Bahit MC, Topol EJ, Califf RM, Armstrong PW, Criger DA, Hasselblad V; et al. (2001). "Reactivation of ischemic events in acute coronary syndromes: results from GUSTO-IIb. Gobal Use of Strategies To Open occluded arteries in acute coronary syndromes". J Am Coll Cardiol. 37 (4): 1001–7. PMID 11263599.
- ↑ Metz BK, White HD, Granger CB, Simes RJ, Armstrong PW, Hirsh J; et al. (1998). "Randomized comparison of direct thrombin inhibition versus heparin in conjunction with fibrinolytic therapy for acute myocardial infarction: results from the GUSTO-IIb Trial. Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes (GUSTO-IIb) Investigators". J Am Coll Cardiol. 31 (7): 1493–8. PMID 9626825.
- ↑ McGuire DK, Emanuelsson H, Granger CB, Magnus Ohman E, Moliterno DJ, White HD; et al. (2000). "Influence of diabetes mellitus on clinical outcomes across the spectrum of acute coronary syndromes. Findings from the GUSTO-IIb study. GUSTO IIb Investigators". Eur Heart J. 21 (21): 1750–8. doi:10.1053/euhj.2000.2317. PMID 11052839.
- ↑ van den Bos AA, Deckers JW, Heyndrickx GR, Laarman GJ, Suryapranata H, Zijlstra F; et al. (1993). "Safety and efficacy of recombinant hirudin (CGP 39 393) versus heparin in patients with stable angina undergoing coronary angioplasty". Circulation. 88 (5 Pt 1): 2058–66. PMID 8222099.
- ↑ Serruys PW, Herrman JP, Simon R, Rutsch W, Bode C, Laarman GJ; et al. (1995). "A comparison of hirudin with heparin in the prevention of restenosis after coronary angioplasty. Helvetica Investigators". N Engl J Med. 333 (12): 757–63. doi:10.1056/NEJM199509213331203. PMID 7643882.
- ↑ "IPRIVASK (desirudin) kit".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Desirudin |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Desirudin |Label Name=Desirudin11.png
}}
{{#subobject:
|Label Page=Desirudin |Label Name=Desirudin11.png
}}