Cyclizine: Difference between revisions
No edit summary |
No edit summary |
||
| (One intermediate revision by the same user not shown) | |||
| Line 21: | Line 21: | ||
*Ask a doctor or pharmacist before use if you have a breathing problem such as [[emphysema]] or [[chronic bronchitis]] glaucoma difficulty in urination due to enlargement of the prostate gland | *Ask a doctor or pharmacist before use if you have a breathing problem such as [[emphysema]] or [[chronic bronchitis]] glaucoma difficulty in urination due to enlargement of the prostate gland | ||
*Ask a doctor before use if you are taking [[sedatives]], or [[tranquilizers]]. | *Ask a doctor before use if you are taking [[sedatives]], or [[tranquilizers]]. | ||
*Do not give this product to children who have a breathing problem such as [[chronic bronchitis]] or who have [[glaucoma]], without first consulting the child's doctor. | |||
Do not give this product to children who have a breathing problem such as chronic bronchitis or who have glaucoma, without first consulting the child's doctor. | *Do not give to children under 6 years of age unless directed by a doctor. | ||
*When using this product you may get drowsy avoid alcohol drinks alcohol, sedatives, and tranquilizers may increase the drowsiness effect be careful when driving a motor vehicle or operating machinery | |||
Do not give to children under 6 years of age unless directed by a doctor. | *In case of overdose, get medical help or contact a Poison Control Center right away. | ||
When using this product you may get drowsy avoid alcohol drinks alcohol, sedatives, and tranquilizers may increase the drowsiness effect be careful when driving a motor vehicle or operating machinery | |||
In case of overdose, get medical help or contact a Poison Control Center right away. | |||
|useInPregnancyFDA=If pregnant or breast-feeding, ask a health professional before use. | |useInPregnancyFDA=If pregnant or breast-feeding, ask a health professional before use. | ||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| Line 83: | Line 79: | ||
| StdInChIKey = UVKZSORBKUEBAZ-UHFFFAOYSA-N | | StdInChIKey = UVKZSORBKUEBAZ-UHFFFAOYSA-N | ||
}} | }} | ||
|structure=[[file:Cyclizine structure.png|none|250px]] | |structure=[[file:Cyclizine structure.png|thumb|none|250px]] | ||
|packLabel=[[file:CyclizinePack14.png|none|400px]] | |packLabel=[[file:CyclizinePack14.png|none|400px]] | ||
|alcohol=Alcohol-Cyclizine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Cyclizine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
Latest revision as of 19:57, 25 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
NOTE: Most over the counter (OTC) are not reviewed and approved by the FDA. However, they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Overview
Cyclizine is an antihistamine that is FDA approved for the treatment of and prevention of nausea, vomiting, or dizziness associated with motion sickness. For the treatment of vertigo of motion sickness. Common adverse reactions include drowsiness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Adults and children 12 years of age and over: 2 tablets every 4-6 hours, not to exceed 8 tablets in 24 hours.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cyclizine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cyclizine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Adults and children 12 years of age and over: 2 tablets every 4-6 hours, not to exceed 8 tablets in 24 hours.
- Children 6 to under 12 years of age: 1 tablet every 6-8 hours, not to exceed 3 tablets in 24 hours.
- Children under 6 years of age: Consult a physician.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Cyclizine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Cyclizine in pediatric patients.
Contraindications
There is limited information regarding Cyclizine Contraindications in the drug label.
Warnings
- Do not exceed recommended dosage.
- Ask a doctor or pharmacist before use if you have a breathing problem such as emphysema or chronic bronchitis glaucoma difficulty in urination due to enlargement of the prostate gland
- Ask a doctor before use if you are taking sedatives, or tranquilizers.
- Do not give this product to children who have a breathing problem such as chronic bronchitis or who have glaucoma, without first consulting the child's doctor.
- Do not give to children under 6 years of age unless directed by a doctor.
- When using this product you may get drowsy avoid alcohol drinks alcohol, sedatives, and tranquilizers may increase the drowsiness effect be careful when driving a motor vehicle or operating machinery
- In case of overdose, get medical help or contact a Poison Control Center right away.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Cyclizine Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Cyclizine Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Cyclizine Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
If pregnant or breast-feeding, ask a health professional before use.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Cyclizine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Cyclizine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Cyclizine in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Cyclizine in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Cyclizine in geriatric settings.
Gender
There is no FDA guidance on the use of Cyclizine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Cyclizine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Cyclizine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Cyclizine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Cyclizine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Cyclizine in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Cyclizine Administration in the drug label.
Monitoring
There is limited information regarding Cyclizine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Cyclizine and IV administrations.
Overdosage
There is limited information regarding Cyclizine overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
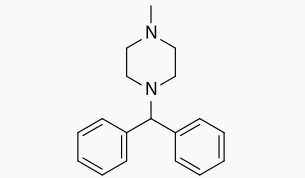
Cyclizine
| |
| Systematic (IUPAC) name | |
| 1-benzhydryl-4-methyl-piperazine | |
| Identifiers | |
| CAS number | |
| ATC code | R06 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 266.381 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | N-demethylated to inactive norcyclizine[citation needed] |
| Half life | 20 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
Pharmacist Only (S3)(AU) P(UK) OTC(US) OTC (Netherlands) |
| Routes | Oral, IM, IV |
Mechanism of Action
There is limited information regarding Cyclizine Mechanism of Action in the drug label.
Structure

Pharmacodynamics
There is limited information regarding Cyclizine Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Cyclizine Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Cyclizine Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Cyclizine Clinical Studies in the drug label.
How Supplied
There is limited information regarding Cyclizine How Supplied in the drug label.
Storage
There is limited information regarding Cyclizine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Cyclizine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Cyclizine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Cyclizine Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Cyclizine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Cyclizine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Cyclizine |Label Name=CyclizinePack1.png
}}
{{#subobject:
|Label Page=Cyclizine |Label Name=CyclizinePack2.png
}}
{{#subobject:
|Label Page=Cyclizine |Label Name=CyclizinePack3.png
}}
| File:Cyclizine.svg | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral, IM, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | N-demethylated to inactive norcyclizine |
| Elimination half-life | 20 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C18H22N2 |
| Molar mass | 266.381 g/mol |
| 3D model (JSmol) | |
| |
- Pages with script errors
- All articles with unsourced statements
- Articles with unsourced statements from October 2011
- Articles with invalid date parameter in template
- Pages with broken file links
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes