Ciprofloxacin (ophthalmic)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ciprofloxacin (ophthalmic) is an antibiotic that is FDA approved for the treatment of bacterial conjunctivitis. Common adverse reactions include blurred vision,allergic reactions,dry eye,edema, hyperemia and keratoconjunctivitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- CILOXAN® (ciprofloxacin hydrochloride ophthalmic ointment) is indicated for the treatment of bacterial conjunctivitis caused by susceptible strains of the microorganisms listed below:
Gram-Positive:
Gram-Negative:
Dosage
- Apply a 1/2" ribbon into the conjunctival sac three times a day on the first two days, then apply a 1/2" ribbon two times a day for the next five days.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ciprofloxacin (ophthalmic) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ciprofloxacin (ophthalmic) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Ciprofloxacin (ophthalmic) in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ciprofloxacin (ophthalmic) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ciprofloxacin (ophthalmic) in pediatric patients.
Contraindications
- A history of hypersensitivity to ciprofloxacin or any other component of the medication is a contraindication to its use. A history of hypersensitivity to other quinolones may also contraindicate the use of ciprofloxacin.
Warnings
FOR TOPICAL OPHTHALMIC USE ONLY.
NOT FOR INJECTION INTO THE EYE.
- Serious and occasionally fatal hypersensitivity (anaphylactic) reactions, some following the first dose, have been reported in patients receiving systemic quinolone therapy. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, tingling, pharyngeal or facial edema, dyspnea, urticaria, and itching. Only a few patients had a history of hypersensitivity reactions. Serious anaphylactic reactions require immediate emergency treatment with epinephrine and other resuscitation measures, including oxygen, intravenous fluids, intravenous antihistamines, corticosteroids, pressor amines and airway management, as clinically indicated.
Adverse Reactions
Clinical Trials Experience
- The following adverse reactions (incidences) were reported in 2% of the patients in clinical studies for CILOXAN® (ciprofloxacin hydrochloride ophthalmic ointment): discomfort, keratopathy. Other reactions associated with ciprofloxacin therapy occurring in less than 1% of patients included allergic reactions, blurred vision, corneal staining, decreased visual acuity, dry eye, edema, epitheliopathy, eye pain, foreign body sensation, hyperemia, irritation, keratoconjunctivitis, lid erythema, lid margin hyperemia, photophobia, pruritus, and tearing.
- Systemic adverse reactions related to ciprofloxacin therapy occurred at an incidence below 1% and included dermatitis, nausea and taste perversion.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Ciprofloxacin (ophthalmic) in the drug label.
Drug Interactions
- Specific drug interaction studies have not been conducted with ophthalmic ciprofloxacin. However, the systemic administration of some quinolones has been shown to elevate plasma concentrations of theophylline, interfere with the metabolism of caffeine, enhance the effects of the oral anticoagulant, warfarin, and its derivatives, and has been associated with transient elevations in serum creatinine in patients receiving cyclosporine concomitantly.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): Pregnancy Category C.
- Reproduction studies have been performed in rats and mice at doses up to six times the usual daily human oral dose and have revealed no evidence of impaired fertility or harm to the fetus due to ciprofloxacin. In rabbits, as with most antimicrobial agents, ciprofloxacin (30 and 100 mg/kg orally) produced gastrointestinal disturbances resulting in maternal weight loss and an increased incidence of abortion. No teratogenicity was observed at either dose. After intravenous administration, at doses up to 20 mg/kg, no maternal toxicity was produced and no embryotoxicity or teratogenicity was observed. There are no adequate and well controlled studies in pregnant women. CILOXAN® (ciprofloxacin hydrochloride ophthalmic ointment) should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ciprofloxacin (ophthalmic) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ciprofloxacin (ophthalmic) during labor and delivery.
Nursing Mothers
- It is not known whether topically applied ciprofloxacin is excreted in human milk. However, it is known that orally administered ciprofloxacin is excreted in the milk of lactating rats and oral ciprofloxacin has been reported in human breast milk after a single 500 mg dose. Caution should be exercised when CILOXAN® (ciprofloxacin hydrochloride ophthalmic ointment) is administered to a nursing mother.
Pediatric Use
- Safety and effectiveness of CILOXAN® (ciprofloxacin hydrochloride ophthalmic ointment) 0.3% in pediatric patients below the age of two years have not been established. Although ciprofloxacin and other quinolones may cause arthropathy in immature Beagle dogs after oral administration, topical ocular administration of ciprofloxacin to immature animals did not cause any arthropathy and there is no evidence that the ophthalmic dosage form has any effect on the weight bearing joints.
Geriatic Use
- No overall clinical differences in safety or effectiveness have been observed between the elderly and other adult patients.
Gender
There is no FDA guidance on the use of Ciprofloxacin (ophthalmic) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ciprofloxacin (ophthalmic) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ciprofloxacin (ophthalmic) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Ciprofloxacin (ophthalmic) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ciprofloxacin (ophthalmic) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ciprofloxacin (ophthalmic) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical
Monitoring
There is limited information regarding Monitoring of Ciprofloxacin (ophthalmic) in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Ciprofloxacin (ophthalmic) in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Ciprofloxacin (ophthalmic) in the drug label.
Pharmacology
Mechanism of Action
- The bactericidal action of ciprofloxacin results from interference with the enzyme DNA gyrase which is needed for the synthesis of bacterial DNA.
Structure
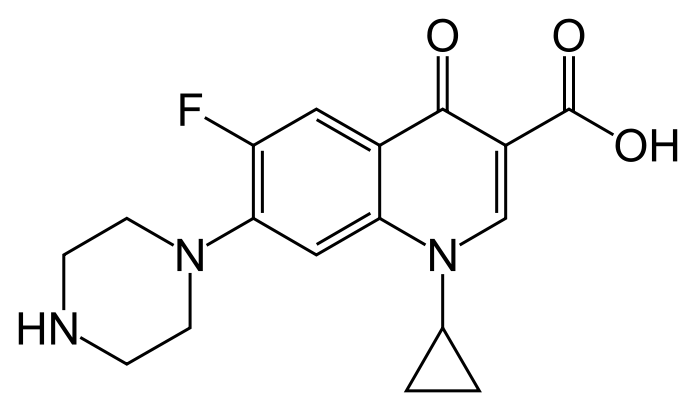
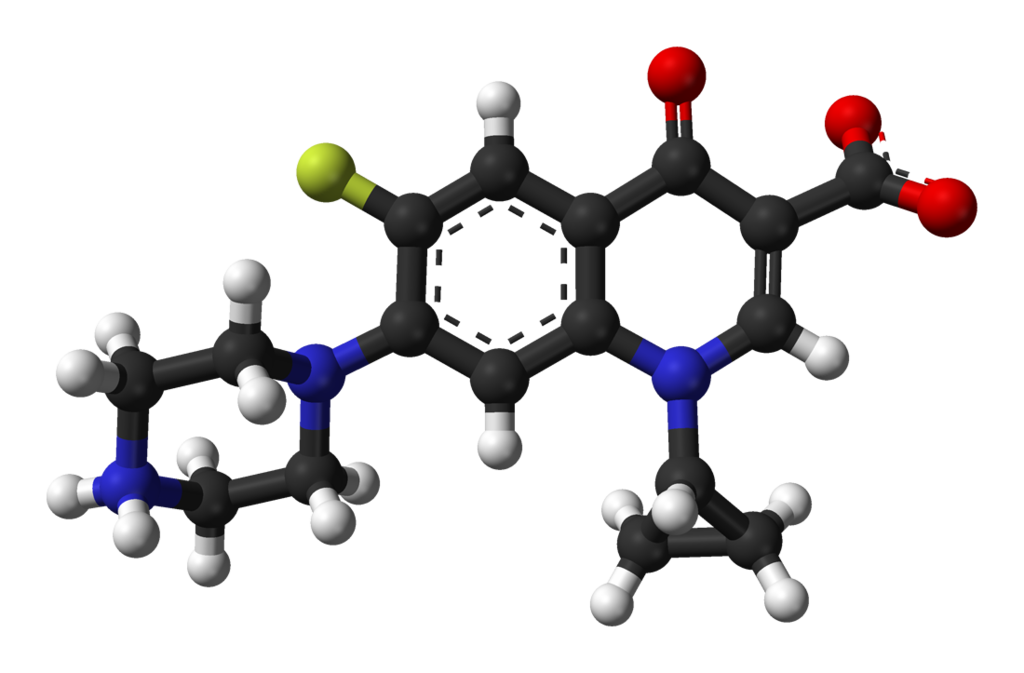
- CILOXAN® (ciprofloxacin hydrochloride ophthalmic ointment) is a synthetic, sterile, multiple dose, antimicrobial for topical use. Ciprofloxacin is a fluoroquinolone antibacterial. It is available as the monohydrochloride monohydrate salt of 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid. Ciprofloxacin is a faint to light yellow crystalline powder with a molecular weight of 385.82. Its empirical formula is C17H18FN3O3•HCl•H2O and its chemical structure is as follows:
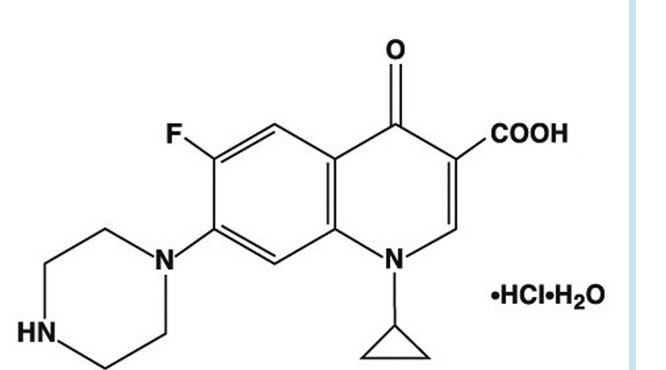
- Ciprofloxacin differs from other quinolones in that it has a fluorine atom at the 6-position, a piperazine moiety at the 7-position, and a cyclopropyl ring at the 1-position.
Each gram of CILOXAN®(ciprofloxacin hydrochloride ophthalmic ointment) contains:
- Active: ciprofloxacin HCl 3.33 mg equivalent to 3 mg base. Inactives: mineral oil, white petrolatum.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Ciprofloxacin (ophthalmic) in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Ciprofloxacin (ophthalmic) in the drug label.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Eight in vitro mutagenicity tests have been conducted with ciprofloxacin and the test results are listed below:
- Salmonella/Microsome Test (Negative)
- E. coli DNA Repair Assay (Negative)
- Mouse Lymphoma Cell Forward Mutation Assay (Positive)
- Chinese Hamster V79 Cell HGPRT Test (Negative)
- Syrian Hamster Embryo Cell Transformation Assay (Negative)
- Saccharomyces cerevisiae Point Mutation Assay (Negative)
- Saccharomyces cerevisiae Mitotic Crossover and Gene Conversion Assay (Negative)
- Rat Hepatocyte DNA Repair Assay (Positive)
- Thus, two of the eight tests were positive, but the results of the following three in vivo test systems gave negative results :
- Rat Hepatocyte DNA Repair Assay
- Micronucleus Test (Mice)
- Dominant Lethal Test (Mice)
- Long-term carcinogenicity studies in mice and rats have been completed. After daily oral dosing for up to two years, there is no evidence that ciprofloxacin had any carcinogenic or tumorigenic effects in these species.
Clinical Studies
- In multicenter clinical trials, approximately 75% of the patients with signs and symptoms of bacterial conjunctivitis and positive conjunctival cultures were clinically cured and approximately 80% had presumed pathogens eradicated by the end of treatment (day 7).
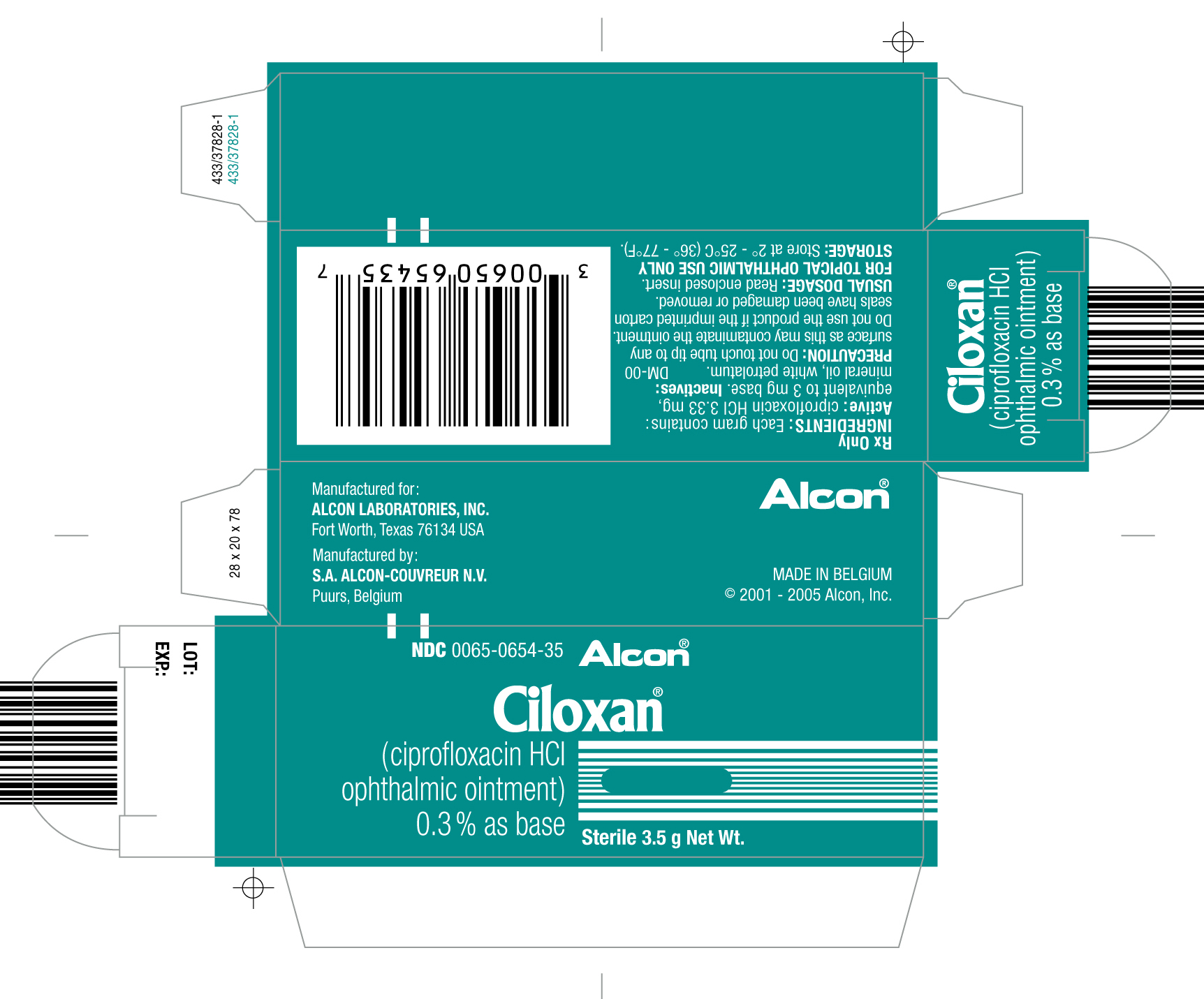
How Supplied
- 3.5 g STERILE ointment supplied in an aluminum tube with a white polyethylene tip and white polyethylene cap. 3.5 g - NDC 0065-0654-35
Storage
- Store at 2° -25°C (36°-77°F).
Images
Drug Images
{{#ask: Page Name::Ciprofloxacin (ophthalmic) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
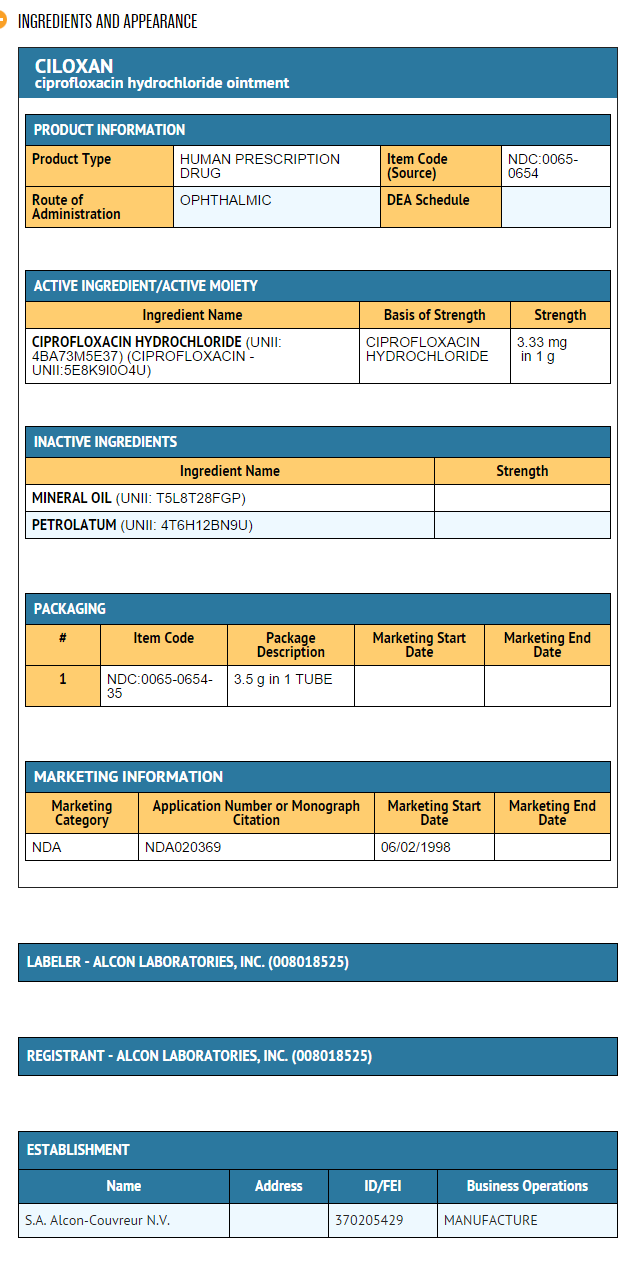
{{#ask: Label Page::Ciprofloxacin (ophthalmic) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Information For Patients
- Do not touch tip to any surface as this may contaminate the ointment.
- Do not use the product if the imprinted carton seals have been damaged, or removed.
Precautions with Alcohol
- Alcohol-Ciprofloxacin (ophthalmic) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CILOXAN®[2]
Look-Alike Drug Names
- A® — B®[3]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Drusano GL, Standiford HC, Plaisance K, Forrest A, Leslie J, Caldwell J, GL (September 1986). "Absolute oral bioavailability of ciprofloxacin". Antimicrob Agents Chemother. 30 (3): 444–6. doi:10.1128/aac.30.3.444. ISSN 0066-4804. PMC 180577. PMID 3777908.
|first2=missing|last2=in Authors list (help);|first3=missing|last3=in Authors list (help);|first4=missing|last4=in Authors list (help);|first5=missing|last5=in Authors list (help);|first6=missing|last6=in Authors list (help) - ↑ "ciprofloxacin hydrochloride ointment".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Ciprofloxacin (ophthalmic)
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Ciprofloxacin (ophthalmic) |Label Name=Ciprofloxacin (ophthalmic)11.png
}}
{{#subobject:
|Label Page=Ciprofloxacin (ophthalmic) |Label Name=Ciprofloxacin (ophthalmic)11.png
}}