Calcitriol receptor
| Vitamin D (1,25- dihydroxyvitamin D3) receptor | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 PDB rendering based on 1kb2. | |||||||||||||
| |||||||||||||
| Identifiers | |||||||||||||
| Symbols | VDR ; NR1I1 | ||||||||||||
| External IDs | Template:OMIM5 Template:MGI HomoloGene: 37297 | ||||||||||||
| |||||||||||||
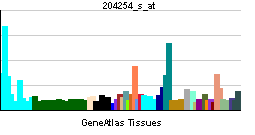
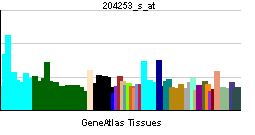
| RNA expression pattern | |||||||||||||
 | |||||||||||||
 | |||||||||||||
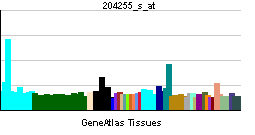 | |||||||||||||
| More reference expression data | |||||||||||||
| Orthologs | |||||||||||||
| Template:GNF Ortholog box | |||||||||||||
| Species | Human | Mouse | |||||||||||
| Entrez | n/a | n/a | |||||||||||
| Ensembl | n/a | n/a | |||||||||||
| UniProt | n/a | n/a | |||||||||||
| RefSeq (mRNA) | n/a | n/a | |||||||||||
| RefSeq (protein) | n/a | n/a | |||||||||||
| Location (UCSC) | n/a | n/a | |||||||||||
| PubMed search | n/a | n/a | |||||||||||
The calcitriol receptor, also known as the Vitamin D receptor (VDR) and also known as NR1I1 (nuclear receptor subfamily 1, group I, member 1), is a member of the steroid hormone receptor family of nuclear receptors.[1] Upon activation by vitamin D, the VDR forms a heterodimer with the retinoid-X receptor and binds to hormone response elements on DNA resulting in expression or transrepression of specific geneproducts.
Glucocorticoids are known to decrease expression of VDR which is expressed in most tissues of the body. Its most well known function is the regulation of intestinal transport of calcium.
This gene encodes the nuclear hormone receptor for vitamin D3. This receptor also functions as a receptor for the secondary bile acid lithocholic acid. The receptor belongs to the family of trans-acting transcriptional regulatory factors and shows sequence similarity to the steroid and thyroid hormone receptors. Downstream targets of this nuclear hormone receptor are principally involved in mineral metabolism though the receptor regulates a variety of other metabolic pathways, such as those involved in the immune response and cancer. Mutations in this gene are associated with type II vitamin D-resistant rickets. A single nucleotide polymorphism in the initiation codon results in an alternate translation start site three codons downstream. Alternative splicing results in multiple transcript variants encoding the same protein.[2]
References
- ↑ Moore DD, Kato S, Xie W, Mangelsdorf DJ, Schmidt DR, Xiao R, Kliewer SA. (2006): "International Union of Pharmacology. LXII. The NR1H and NR1I receptors: constitutive androstane receptor, pregnene X receptor, farnesoid X receptor alpha, farnesoid X receptor beta, liver X receptor alpha, liver X receptor beta, and vitamin D receptor." Pharmacol Rev. 58(4):742-759. PMID 17132852
- ↑ "Entrez Gene: VDR vitamin D (1,25- dihydroxyvitamin D3) receptor".
Further reading
- Hosoi T (2002). "[Polymorphisms of vitamin D receptor gene]". Nippon Rinsho. 60 Suppl 3: 106–10. PMID 11979895.
- Uitterlinden AG, Fang Y, Van Meurs JB; et al. (2004). "Genetics and biology of vitamin D receptor polymorphisms". Gene. 338 (2): 143–56. doi:10.1016/j.gene.2004.05.014. PMID 15315818.
- Norman AW (2007). "Minireview: vitamin D receptor: new assignments for an already busy receptor". Endocrinology. 147 (12): 5542–8. doi:10.1210/en.2006-0946. PMID 16946007.
- Bollag WB (2007). "Differentiation of human keratinocytes requires the vitamin d receptor and its coactivators". J. Invest. Dermatol. 127 (4): 748–50. doi:10.1038/sj.jid.5700692. PMID 17363957.
- Bugge TH, Pohl J, Lonnoy O, Stunnenberg HG (1992). "RXR alpha, a promiscuous partner of retinoic acid and thyroid hormone receptors". EMBO J. 11 (4): 1409–18. PMID 1314167.
- Goto H, Chen KS, Prahl JM, DeLuca HF (1992). "A single receptor identical with that from intestine/T47D cells mediates the action of 1,25-dihydroxyvitamin D-3 in HL-60 cells". Biochim. Biophys. Acta. 1132 (1): 103–8. PMID 1324736.
- Saijo T, Ito M, Takeda E; et al. (1991). "A unique mutation in the vitamin D receptor gene in three Japanese patients with vitamin D-dependent rickets type II: utility of single-strand conformation polymorphism analysis for heterozygous carrier detection". Am. J. Hum. Genet. 49 (3): 668–73. PMID 1652893.
- Szpirer J, Szpirer C, Riviere M; et al. (1992). "The Sp1 transcription factor gene (SP1) and the 1,25-dihydroxyvitamin D3 receptor gene (VDR) are colocalized on human chromosome arm 12q and rat chromosome 7". Genomics. 11 (1): 168–73. PMID 1662663.
- Yu XP, Mocharla H, Hustmyer FG, Manolagas SC (1991). "Vitamin D receptor expression in human lymphocytes. Signal requirements and characterization by western blots and DNA sequencing". J. Biol. Chem. 266 (12): 7588–95. PMID 1850412.
- Malloy PJ, Hochberg Z, Tiosano D; et al. (1991). "The molecular basis of hereditary 1,25-dihydroxyvitamin D3 resistant rickets in seven related families". J. Clin. Invest. 86 (6): 2071–9. PMID 2174914.
- Sone T, Marx SJ, Liberman UA, Pike JW (1991). "A unique point mutation in the human vitamin D receptor chromosomal gene confers hereditary resistance to 1,25-dihydroxyvitamin D3". Mol. Endocrinol. 4 (4): 623–31. PMID 2177843.
- Baker AR, McDonnell DP, Hughes M; et al. (1988). "Cloning and expression of full-length cDNA encoding human vitamin D receptor". Proc. Natl. Acad. Sci. U.S.A. 85 (10): 3294–8. PMID 2835767.
- Hughes MR, Malloy PJ, Kieback DG; et al. (1989). "Point mutations in the human vitamin D receptor gene associated with hypocalcemic rickets". Science. 242 (4886): 1702–5. PMID 2849209.
- Rut AR, Hewison M, Kristjansson K; et al. (1995). "Two mutations causing vitamin D resistant rickets: modelling on the basis of steroid hormone receptor DNA-binding domain crystal structures". Clin. Endocrinol. (Oxf). 41 (5): 581–90. PMID 7828346.
- Malloy PJ, Weisman Y, Feldman D (1994). "Hereditary 1 alpha,25-dihydroxyvitamin D-resistant rickets resulting from a mutation in the vitamin D receptor deoxyribonucleic acid-binding domain". J. Clin. Endocrinol. Metab. 78 (2): 313–6. PMID 8106618.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. PMID 8125298.
- Yagi H, Ozono K, Miyake H; et al. (1993). "A new point mutation in the deoxyribonucleic acid-binding domain of the vitamin D receptor in a kindred with hereditary 1,25-dihydroxyvitamin D-resistant rickets". J. Clin. Endocrinol. Metab. 76 (2): 509–12. PMID 8381803.
- Kristjansson K, Rut AR, Hewison M; et al. (1993). "Two mutations in the hormone binding domain of the vitamin D receptor cause tissue resistance to 1,25 dihydroxyvitamin D3". J. Clin. Invest. 92 (1): 12–6. PMID 8392085.
- Jurutka PW, Hsieh JC, Nakajima S; et al. (1996). "Human vitamin D receptor phosphorylation by casein kinase II at Ser-208 potentiates transcriptional activation". Proc. Natl. Acad. Sci. U.S.A. 93 (8): 3519–24. PMID 8622969.
- Lin NU, Malloy PJ, Sakati N; et al. (1996). "A novel mutation in the deoxyribonucleic acid-binding domain of the vitamin D receptor causes hereditary 1,25-dihydroxyvitamin D-resistant rickets". J. Clin. Endocrinol. Metab. 81 (7): 2564–9. PMID 8675579.
External links
- Calcitriol+Receptors at the US National Library of Medicine Medical Subject Headings (MeSH)
This article incorporates text from the United States National Library of Medicine, which is in the public domain.
| Stub icon | This genetics article is a stub. You can help Wikipedia by expanding it. |