Babesiosis pathophysiology: Difference between revisions
| Line 11: | Line 11: | ||
===Pathogenesis=== | ===Pathogenesis=== | ||
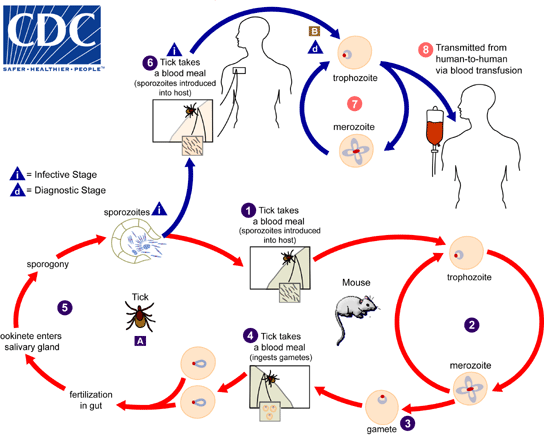
The ''Babesia microti'' life cycle involves two | The ''Babesia microti'' life cycle involves two primary components, an infected vertebrate host (primarily the white-footed mouse "Peromyscus leucopus"), and a tick in the genus ''Ixodes''. <ref>http://www.cdc.gov/parasites/babesiosis/biology.html</ref> | ||
1. During a blood meal, a ''Babesia''-infected tick introduces [[sporozoites]] into the mouse host. <br> | 1. During a blood meal, a ''Babesia''-infected tick introduces [[sporozoites]] into the mouse host. <br> | ||
| Line 19: | Line 19: | ||
*Transovarial transmission (also known as vertical, or hereditary, transmission) has been documented for "large" ''Babesia'' spp. but not for the "small" babesiae, such as ''B. microti'' (A). <br> | *Transovarial transmission (also known as vertical, or hereditary, transmission) has been documented for "large" ''Babesia'' spp. but not for the "small" babesiae, such as ''B. microti'' (A). <br> | ||
6. Humans enter the cycle when bitten by infected ticks. During a blood meal, a Babesia-infected [[tick]] introduces sporozoites into the human host.<br> | 6. Humans enter the cycle when bitten by infected ticks. During a blood meal, a Babesia-infected [[tick]] introduces sporozoites into the human host.<br> | ||
7. Sporozoites enter erythrocytes (B) and undergo | 7. Sporozoites enter erythrocytes (B) and undergo (budding). <br> | ||
8. Multiplication of the blood-stage [[parasites]] is responsible for the clinical manifestations of the disease. Humans are usually dead-end hosts. However, human-to-human transmission is well recognized to occur via contaminated [[blood transfusions]].<br> | 8. Multiplication of the blood-stage [[parasites]] is responsible for the clinical manifestations of the disease. Humans are usually dead-end hosts. However, human-to-human transmission is well recognized to occur via contaminated [[blood transfusions]].<br> | ||
Revision as of 15:54, 4 December 2015
|
Babesiosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Babesiosis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Babesiosis pathophysiology |
|
Risk calculators and risk factors for Babesiosis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Tamar Sifri [2]
Overview
Babesia parasites reproduce in red blood cells, where they can be seen as cross-shaped inclusions (4 merozoites asexually budding but attached together forming a structure looking like a "Maltese Cross") and cause hemolytic anemia, quite similar to malaria.
Note that unlike the Plasmodium parasites that cause malaria, Babesia species lack an exo-erythrotic phase, so the liver is usually not affected.
Pathophysiology
Pathogenesis
The Babesia microti life cycle involves two primary components, an infected vertebrate host (primarily the white-footed mouse "Peromyscus leucopus"), and a tick in the genus Ixodes. [1]
1. During a blood meal, a Babesia-infected tick introduces sporozoites into the mouse host.
2. Sporozoites enter erythrocytes and undergo asexual reproduction (budding).
3. In the blood, some parasites differentiate into male and female gametes, although these cannot be distinguished by light microscopy.
4. The definitive host is the tick. Once ingested by an appropriate tick, gametes unite and undergo a sporogonic cycle resulting in sporozoites (5).
- Transovarial transmission (also known as vertical, or hereditary, transmission) has been documented for "large" Babesia spp. but not for the "small" babesiae, such as B. microti (A).
6. Humans enter the cycle when bitten by infected ticks. During a blood meal, a Babesia-infected tick introduces sporozoites into the human host.
7. Sporozoites enter erythrocytes (B) and undergo (budding).
8. Multiplication of the blood-stage parasites is responsible for the clinical manifestations of the disease. Humans are usually dead-end hosts. However, human-to-human transmission is well recognized to occur via contaminated blood transfusions.
-
Life cycle of B. microti
Adapted from CDC