Amoxicillin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Amoxicillin is an antibiotic that is FDA approved for the treatment of infections of the ear, nose, throat, genitourinary tract and lower respiratory tract. Also for Gonorrhea and helicobacter infections. Common adverse reactions include rash, diarrhea, nausea, headache and vulvovaginitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
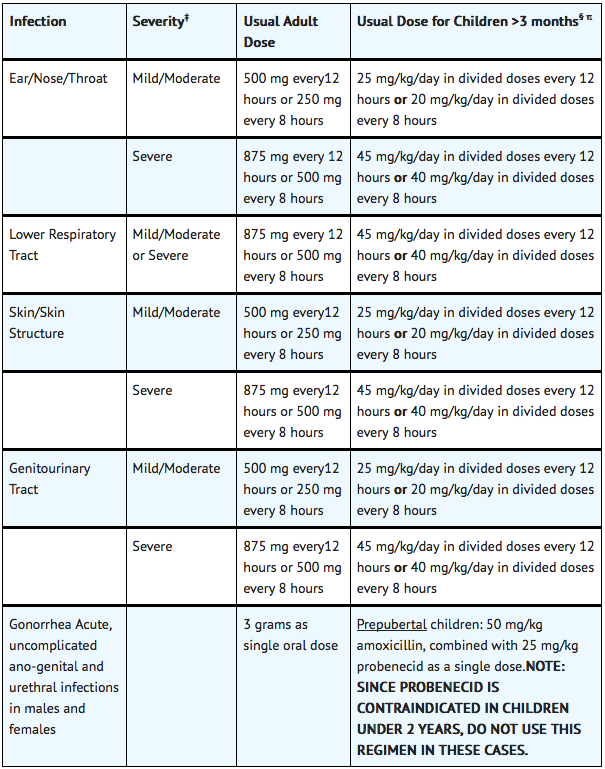
§ The children’s dosage is intended for individuals whose weight is less than 40 kg. Children weighing 40 kg or more should be dosed according to the adult recommendations.
π Each strength of the suspension of amoxicillin is available as a chewable tablet for use by older children.
H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence:
- Triple Therapy:
- Amoxicillin
- Clarithromycin
- Lansoprazole.
The recommended adult oral dose is 1 gram amoxicillin, 500 mg clarithromycin, and 30 mg lansoprazole, all given twice daily (q12h) for 14 days.
Community Acquired Pneumonia
- In patients wit comorbidities or use of antimicrobials within the previous 3 month
- Dosage: 875mg PO q12h OR 500mg q8h
Acute Bacterial Rhinosinusitis
- Mild/Moderate: 500mg q12h or 250mg q8h
- Severe: 875mg q12h or 500mg q8h
Gonorrhea, Acute uncomplicated anogenital and urethral infections due to Neisseria gonorrhoeae
- Dosage: 3g as single oral dose
Helicobacter Pylori infection
- Triple Therapy for 14 days
- Amoxicillin: 1g q12h PO
- Clarithromycin: 500mg q12h
- Lansoprazole: 30mg q12h
- Dual Therapy
- Amoxicillin: 1g q8h
- Lansoprazole: 30mg q8h
Infection of skin AND/OR subcutaneous tissue
- Mild/moderate: 500mg q12h or 250mg q8h
- Severe: 875mg q12h or 500mg q8h
Infectious disease of genitourinary system
- Mild/Moderate: 500 mg q12h or 250mg q8h
- Severe: 875mg q8h or 500mg q8h
Lower Respiratory Tract Infection
- Dosage: 875 mg q12h or 500 mg q8h
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Bacterial Endocarditis Prophylaxis
- Dosage: 2g PO q24h [1]
Chlamydial Infection
- Pregnant women: 500mg q8h PO for 7 days. [2]
Lyme's Disease
- Eritema migrans dosage: 500mg PO TID for 14-21 days
- Seventh-cranial nerve palsy: 500mg PO TID for 14-21 days
- Lyme's arthritis: 500mg PO TID for 14-21 days[3]
Non–Guideline-Supported Use
Actinomycotic Infection
- Dosage: 500mg q8h PO (in combination with cotrimoxazole (80mg/400mg) 2 tablets q12h for 2-5 months for home regimen) [4]
Acute Infective Exacerbation of Chronic Obstructive Pulmonary Disease
- Dosage: 1g q12h PO. [5]
Cutaneous Anthrax
- When first-line drugs are contraindicated in the patient
- Dosage: 1g q8h PO [6]
Periodontal infection
- Dosage: 500mg Amoxicillin q8h in combination with metronidazole 250-400mg q8h[7]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Otitis Media with Effusion
- Mild/Moderate: 25 mg/kg/day in divided doses q12h OR 20 mg/kg/day in divided doses q8h
- Severe: 45 mg/kg/day in divided doses q12h OR 40 mg/kg/day in divided doses q8h
Acute Bacterial Rhinosinusitis
- Mild/Moderate: 25 mg/kg/day in divided doses q12h OR 20 mg/kg/day in divided doses q8h
- Severe: 45 mg/kg/day in divided doses q12h OR 40 mg/kg/day in divided doses q8h
Gonorrhea, Acute uncomplicated anogenital and urethral infections due to Neisseria gonorrhoeae
- Dosage: 50 mg/kg Amoxicillin combined with 25 mg/kg Probenecid (Only in pediatric population >2 years old).
Infection of skin AND/OR subcutaneous tissue
- Mild/Moderate: 25mg/kg/day in divided doses q12h or 20mg/kg/day in divided doses q8h
- Severe: 45mg/kg/day in divided doses q12h or 40mg/kg/day in divided doses q8h
Infectious disease of genitourinary system
- Mild/Moderate: 25mg/kg/day in divided doses q12h or 20mg/kg/day in divided doses q8h
- Severe: 45mg/kg/day in divided doses q12h or 40 mg/kg/day in divided doses q8h
Lower Respiratory Tract Infection
- Dosage: 45mg/kg/day in divided doses q12h or 40mg/kg/day in divided doses q8h
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Lyme's Disease
- Eritema migrans dosage: 50mg/kg/day PO in 3 divided doses (Max: 1500mg)
- Lyme's arthritis: 50 mg/kg/day PO in 3 divided doses (Max: 1500mg)
- Seventh-cranial nerve palsy: 50 mg/kg/day PO in 3 divided doses (Max: 1500mg)[3]
Non–Guideline-Supported Use
Periodontal infection
- Dosage: 500mg Amoxicillin q8h in combination with metronidazole 250-400mg q8h[7]
Contraindications
There is limited information regarding Amoxicillin Contraindications in the drug label.
Warnings
There is limited information regarding Amoxicillin Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Amoxicillin Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Amoxicillin Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Amoxicillin Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Amoxicillin in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Amoxicillin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Amoxicillin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Amoxicillin in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Amoxicillin in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Amoxicillin in geriatric settings.
Gender
There is no FDA guidance on the use of Amoxicillin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Amoxicillin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Amoxicillin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Amoxicillin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Amoxicillin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Amoxicillin in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Amoxicillin Administration in the drug label.
Monitoring
There is limited information regarding Amoxicillin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Amoxicillin and IV administrations.
Overdosage
There is limited information regarding Amoxicillin overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Amoxicillin Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Amoxicillin Mechanism of Action in the drug label.
Structure
There is limited information regarding Amoxicillin Structure in the drug label.
Pharmacodynamics
There is limited information regarding Amoxicillin Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Amoxicillin Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Amoxicillin Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Amoxicillin Clinical Studies in the drug label.
How Supplied
There is limited information regarding Amoxicillin How Supplied in the drug label.
Storage
There is limited information regarding Amoxicillin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Amoxicillin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Amoxicillin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Amoxicillin Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Amoxicillin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Amoxicillin Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Amoxicillin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M; et al. (2007). "Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group". Circulation. 116 (15): 1736–54. doi:10.1161/CIRCULATIONAHA.106.183095. PMID 17446442.
- ↑ "Centers for Disease Control and Prevention: Sexually transmitted diseases treatment guidelines, 2010" (PDF).
- ↑ 3.0 3.1 Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS; et al. (2006). "The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America". Clin Infect Dis. 43 (9): 1089–134. doi:10.1086/508667. PMID 17029130.
- ↑ Ramam M, Garg T, D'Souza P, Verma KK, Khaitan BK, Singh MK; et al. (2000). "A two-step schedule for the treatment of actinomycotic mycetomas". Acta Derm Venereol. 80 (5): 378–80. PMID 11200840.
- ↑ Georgopoulos A, Borek M, Ridl W, Amoxycillin Bronchitis Study Group (2001). "Randomized, double-blind, double-dummy study comparing the efficacy and safety of amoxycillin 1 g bd with amoxycillin 500 mg tds in the treatment of acute exacerbations of chronic bronchitis". J Antimicrob Chemother. 47 (1): 67–76. PMID 11152433.
- ↑ Inglesby TV, O'Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E; et al. (2002). "Anthrax as a biological weapon, 2002: updated recommendations for management". JAMA. 287 (17): 2236–52. PMID 11980524.
- ↑ 7.0 7.1 Sgolastra F, Gatto R, Petrucci A, Monaco A (2012). "Effectiveness of systemic amoxicillin/metronidazole as adjunctive therapy to scaling and root planing in the treatment of chronic periodontitis: a systematic review and meta-analysis". J Periodontol. 83 (10): 1257–69. doi:10.1902/jop.2012.110625. PMID 22220767.
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_01439951.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN]|+sep=; |Pill Imprint=WW;951 |Pill Dosage=875 mg |Pill Color=Pink|+sep=; |Pill Shape=Oval |Pill Size (mm)=9 |Pill Scoring=2 |Pill Image= |Drug Author=West-ward Pharmaceutical Corp |NDC=01439951
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_and_Clavulanate_Potassium_NDC_07811619.jpg |Drug Name=Amoxicillin and Clavulanate Potassium |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=GGN2 |Pill Dosage=200 mg |Pill Color=Pink|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=1 |Pill Image= |Drug Author=Sandoz Inc |NDC=07811619
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_07812613.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=AMOX;500;GG;849 |Pill Dosage=500 mg |Pill Color=Yellow|+sep=; |Pill Shape=Capsule |Pill Size (mm)=22 |Pill Scoring=1 |Pill Image= |Drug Author=Sandoz Inc |NDC=07812613
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_422910120.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=AA;820 |Pill Dosage=250 mg |Pill Color=Brown|+sep=; |Pill Shape=Capsule |Pill Size (mm)=18 |Pill Scoring=1 |Pill Image= |Drug Author=AvKARE, Inc. |NDC=422910120
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_422910121.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=AA;825 |Pill Dosage=500 mg |Pill Color=Brown|+sep=; |Pill Shape=Capsule |Pill Size (mm)=22 |Pill Scoring=1 |Pill Image= |Drug Author=AvKARE, Inc. |NDC=422910121
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=AUGMENTIN_NDC_435980020.jpg |Drug Name=AUGMENTIN |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];AMOXICILLIN SODIUM[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=AUGMENTIN;XR |Pill Dosage=562.5 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=22 |Pill Scoring=2 |Pill Image= |Drug Author=Dr Reddys Laboratories Inc |NDC=435980020
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=amoxicillin_and_clavulanate_potassium_NDC_435980206.jpg |Drug Name=amoxicillin and clavulanate potassium |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=AUGMENTIN;500;125; |Pill Dosage=500 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=18 |Pill Scoring=1 |Pill Image= |Drug Author=Dr. Reddy's Laboratories Inc |NDC=435980206
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=amoxicillin_and_clavulanate_potassium_NDC_435980206.jpg |Drug Name=amoxicillin and clavulanate potassium |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=AUGMENTIN;500;125; |Pill Dosage=500 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=18 |Pill Scoring=1 |Pill Image= |Drug Author=Dr. Reddy's Laboratories Inc |NDC=435980206
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=amoxicillin_and_clavulanate_potassium_NDC_435980218.jpg |Drug Name=amoxicillin and clavulanate potassium |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=AUGMENTIN;250;125; |Pill Dosage=250 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=18 |Pill Scoring=1 |Pill Image= |Drug Author=Dr. Reddy's Laboratories Inc |NDC=435980218
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_and_Clavulanate_Potassium_NDC_435980220.jpg |Drug Name=Amoxicillin and Clavulanate Potassium |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];AMOXICILLIN SODIUM[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=AUGMENTIN;XR |Pill Dosage=562.5 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=22 |Pill Scoring=2 |Pill Image= |Drug Author=Dr Reddys Laboratories Inc |NDC=435980220
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=amoxicillin_and_clavulanate_potassium_NDC_435980221.jpg |Drug Name=amoxicillin and clavulanate potassium |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=AUGMENTIN;875 |Pill Dosage=875 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=18 |Pill Scoring=2 |Pill Image= |Drug Author=Dr. Reddy's Laboratories Inc |NDC=435980221
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_604290021.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=WC;730; |Pill Dosage=250 mg |Pill Color=Orange|+sep=; |Pill Shape=Capsule |Pill Size (mm)=16 |Pill Scoring=1 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290021
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_604290022.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=WC;731 |Pill Dosage=500 mg |Pill Color=Orange|+sep=; |Pill Shape=Capsule |Pill Size (mm)=16 |Pill Scoring=1 |Pill Image= |Drug Author=Golden State Medical Supply, Inc. |NDC=604290022
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=AMOXICILLIN_NDC_633040763.jpg |Drug Name=AMOXICILLIN |Pill Ingred=amoxicillin[amoxicillin]|+sep=; |Pill Imprint=RX763 |Pill Dosage=875 mg |Pill Color=Pink|+sep=; |Pill Shape=Oval |Pill Size (mm)=21 |Pill Scoring=2 |Pill Image= |Drug Author=Ranbaxy Pharmaceutical Inc. |NDC=633040763
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_658620015.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=A;6;7 |Pill Dosage=875 mg |Pill Color=Pink|+sep=; |Pill Shape=Oval |Pill Size (mm)=21 |Pill Scoring=2 |Pill Image= |Drug Author=Aurobindo Pharma Limited |NDC=658620015
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_and_Clavulanate_Potassium_NDC_666851001.jpg |Drug Name=Amoxicillin and Clavulanate Potassium |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=875125;AMC |Pill Dosage=875 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=13 |Pill Scoring=2 |Pill Image= |Drug Author=Sandoz Inc |NDC=666851001
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_and_Clavulanate_Potassium_NDC_666851002.jpg |Drug Name=Amoxicillin and Clavulanate Potassium |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=500125;AMC |Pill Dosage=500 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=13 |Pill Scoring=1 |Pill Image= |Drug Author=Sandoz Inc |NDC=666851002
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_00932263.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=93;2263 |Pill Dosage=500 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=17 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00932263
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_00932264.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=93;2264 |Pill Dosage=875 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=21 |Pill Scoring=2 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00932264
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_and_Clavulanate_Potassium_NDC_07811643.jpg |Drug Name=Amoxicillin and Clavulanate Potassium |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=GGN4 |Pill Dosage=400 mg |Pill Color=Pink|+sep=; |Pill Shape=Round |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Sandoz Inc |NDC=07811643
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_and_Clavulanate_Potassium_NDC_07811943.jpg |Drug Name=Amoxicillin and Clavulanate Potassium |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];AMOXICILLIN SODIUM[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=SZ137 |Pill Dosage=562.5 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=22 |Pill Scoring=2 |Pill Image= |Drug Author=Sandoz Inc |NDC=07811943
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_07812020.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=AMOX;250;GG;848 |Pill Dosage=250 mg |Pill Color=Yellow|+sep=; |Pill Shape=Capsule |Pill Size (mm)=18 |Pill Scoring=1 |Pill Image= |Drug Author=Sandoz Inc |NDC=07812020
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_07815061.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=GG;962;875 |Pill Dosage=875 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=21 |Pill Scoring=2 |Pill Image= |Drug Author=Sandoz Inc |NDC=07815061
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_545693689.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=9;3;2268 |Pill Dosage=250 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=2 |Pill Image= |Drug Author=A-S Medication Solutions LLC |NDC=545693689
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_661160821.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=TEVA;3109 |Pill Dosage=500 mg |Pill Color=Brown|+sep=; |Pill Shape=Capsule |Pill Size (mm)=22 |Pill Scoring=1 |Pill Image= |Drug Author=MedVantx, Inc. |NDC=661160821
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_00932267.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=93;2267 |Pill Dosage=125 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=15 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00932267
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_00932268.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=9;3;2268 |Pill Dosage=250 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=19 |Pill Scoring=2 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00932268
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_00933107.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=TEVA;3107 |Pill Dosage=250 mg |Pill Color=|+sep=; |Pill Shape=Capsule |Pill Size (mm)=18 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00933107
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_00933109.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=TEVA;3109 |Pill Dosage=500 mg |Pill Color=Brown|+sep=; |Pill Shape=Capsule |Pill Size (mm)=22 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00933109
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_597621020.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=A44 |Pill Dosage=250 mg |Pill Color=|+sep=; |Pill Shape=Capsule |Pill Size (mm)=19 |Pill Scoring=1 |Pill Image= |Drug Author=Greenstone LLC |NDC=597621020
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_597621050.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=A;6;7 |Pill Dosage=875 mg |Pill Color=Pink|+sep=; |Pill Shape=Oval |Pill Size (mm)=21 |Pill Scoring=2 |Pill Image= |Drug Author=Greenstone LLC |NDC=597621050
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_552890020.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=TEVA;3109 |Pill Dosage=500 ug |Pill Color=Brown|+sep=; |Pill Shape=Capsule |Pill Size (mm)=22 |Pill Scoring=1 |Pill Image= |Drug Author=PD-Rx Pharmaceuticals, Inc. |NDC=552890020
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_658620017.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=A45 |Pill Dosage=500 mg |Pill Color=|+sep=; |Pill Shape=Capsule |Pill Size (mm)=23 |Pill Scoring=1 |Pill Image= |Drug Author=Aurobindo Pharma Limited |NDC=658620017
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_672530141.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=WC;731 |Pill Dosage=500 mg |Pill Color=|+sep=; |Pill Shape=Capsule |Pill Size (mm)=23 |Pill Scoring=1 |Pill Image= |Drug Author=DAVA Pharmaceuticals, Inc. |NDC=672530141
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Moxatag_NDC_684530142.jpg |Drug Name=Moxatag |Pill Ingred=amoxicillin[amoxicillin]|+sep=; |Pill Imprint=MB;111 |Pill Dosage=775 mg |Pill Color=Blue|+sep=; |Pill Shape=Oval |Pill Size (mm)=22 |Pill Scoring=1 |Pill Image= |Drug Author=Victory Pharma, Inc. |NDC=684530142
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_NDC_430630434.jpg |Drug Name=Amoxicillin |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS]|+sep=; |Pill Imprint=TEVA;3109 |Pill Dosage=500 mg |Pill Color=Brown|+sep=; |Pill Shape=Capsule |Pill Size (mm)=22 |Pill Scoring=1 |Pill Image= |Drug Author=PD-Rx Pharmaceuticals, Inc. |NDC=430630434
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_and_Clavulanate_Potassium_NDC_00932270.jpg |Drug Name=Amoxicillin and Clavulanate Potassium |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=93;2270 |Pill Dosage=200 mg |Pill Color=Pink|+sep=; |Pill Shape=Oval |Pill Size (mm)=16 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00932270
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_and_Clavulanate_Potassium_NDC_00932272.jpg |Drug Name=Amoxicillin and Clavulanate Potassium |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=93;2272 |Pill Dosage=400 mg |Pill Color=Pink|+sep=; |Pill Shape=Oval |Pill Size (mm)=21 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00932272
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_and_Clavulanate_Potassium_NDC_00932274.jpg |Drug Name=Amoxicillin and Clavulanate Potassium |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=93;2274 |Pill Dosage=500 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=20 |Pill Scoring=1 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00932274
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_and_Clavulanate_Potassium_NDC_00932275.jpg |Drug Name=Amoxicillin and Clavulanate Potassium |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=93;22;75 |Pill Dosage=875 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=22 |Pill Scoring=2 |Pill Image= |Drug Author=Teva Pharmaceuticals USA Inc |NDC=00932275
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_and_Clavulanate_Potassium_NDC_07811874.jpg |Drug Name=Amoxicillin and Clavulanate Potassium |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=GGN5 |Pill Dosage=250 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=18 |Pill Scoring=1 |Pill Image= |Drug Author=Sandoz Inc |NDC=07811874
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_and_Clavulanate_Potassium_NDC_07811874.jpg |Drug Name=Amoxicillin and Clavulanate Potassium |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=GGN5 |Pill Dosage=250 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=18 |Pill Scoring=1 |Pill Image= |Drug Author=Sandoz Inc |NDC=07811874
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=AUGMENTIN_NDC_435980020.jpg |Drug Name=AUGMENTIN |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];AMOXICILLIN SODIUM[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=AUGMENTIN;XR |Pill Dosage=562.5 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=22 |Pill Scoring=2 |Pill Image= |Drug Author=Dr Reddys Laboratories Inc |NDC=435980020
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_and_Clavulanate_Potassium_NDC_435980220.jpg |Drug Name=Amoxicillin and Clavulanate Potassium |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];AMOXICILLIN SODIUM[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=AUGMENTIN;XR |Pill Dosage=562.5 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=22 |Pill Scoring=2 |Pill Image= |Drug Author=Dr Reddys Laboratories Inc |NDC=435980220
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=Amoxicillin_and_Clavulanate_Potassium_NDC_07811943.jpg |Drug Name=Amoxicillin and Clavulanate Potassium |Pill Ingred=AMOXICILLIN[AMOXICILLIN ANHYDROUS];AMOXICILLIN SODIUM[AMOXICILLIN ANHYDROUS];CLAVULANATE POTASSIUM[CLAVULANIC ACID]|+sep=; |Pill Imprint=SZ137 |Pill Dosage=562.5 mg |Pill Color=White|+sep=; |Pill Shape=Oval |Pill Size (mm)=22 |Pill Scoring=2 |Pill Image= |Drug Author=Sandoz Inc |NDC=07811943
}}
{{#subobject:
|Page Name=Amoxicillin |Pill Name=MOXATAG_NDC_110420142.jpg |Drug Name=MOXATAG |Pill Ingred=amoxicillin[amoxicillin]|+sep=; |Pill Imprint=MB-111 |Pill Dosage=775 mg |Pill Color=Blue|+sep=; |Pill Shape=Oval |Pill Size (mm)=22 |Pill Scoring=1 |Pill Image= |Drug Author=MiddleBrook Pharmaceuticals, Inc. |NDC=110420142
}}