Aminoglutethimide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Overview
Aminoglutethimide is a that is FDA approved for the {{{indicationType}}} of . There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Cytadren is indicated for the suppression of adrenal function in selected patients with Cushing’s syndrome. Morning levels of plasma cortisol in patients with adrenal carcinoma and ectopic ACTHproducing tumors were reduced on the average to about one half of the pretreatment levels, and in patients with adrenal hyperplasia to about two thirds of the pretreatment levels, during 1-3 months of therapy with Cytadren. Data available from the few patients with adrenal adenoma suggest similar reductions in plasma cortisol levels. Measurements of plasma cortisol showed reductions to at least 50% of baseline or to normal levels in one third or more of the patients studied, depending on diagnostic groups and time of measurement.
- Because Cytadren does not affect the underlying disease process, it is used primarily as an interim measure until more definitive therapy such as surgery can be undertaken or in cases where such therapy is not appropriate. Only small numbers of patients have been treated for longer than 3 months. A decreased effect or “escape phenomenon” seems to occur more frequently in patients with pituitarydependent Cushing’s syndrome, probably because of increasing ACTH levels in response to decreasing glucocorticoid levels.
- Cytadren should be used only in those patients who are responsive to treatment.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
There is limited information regarding Off-Label Guideline-Supported Use of Aminoglutethimide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aminoglutethimide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Aminoglutethimide in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aminoglutethimide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aminoglutethimide in pediatric patients.
Contraindications
- Cytadren is contraindicated in those patients with serious forms, and/or more severe manifestations, of hypersensitivity to glutethimide or aminoglutethimide.
Warnings
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
- Cytadren may cause adrenocortical hypofunction, especially under conditions of stress, such as surgery, trauma, or acute illness. Patients should be carefully monitored and given hydrocortisone and mineralocorticoid supplements as indicated. Dexamethasone should not be used.
- Cytadren also may suppress aldosterone production by the adrenal cortex and may cause orthostatic or persistent hypotension. The blood pressure should be monitored in all patients at appropriate intervals. Patients should be advised of the possible occurrence of weakness and dizziness as symptoms of hypotension, and of measures to be taken should they occur. The effects of Cytadren may be potentiated if it is taken in combination with alcohol. Cytadren can cause fetal harm when administered to a pregnant woman. In the earlier experience with the drug in about 5000 patients, two cases of pseudohermaphroditism were reported in female infants whose mothers were treated with Cytadren and concomitant anticonvulsants. Normal pregnancies have also occurred in patients treated with Cytadren. When administered to rats at doses 1/2 and 1 1/4 times the maximum daily human dose, Cytadren caused a decrease in fetal implantation, an increase in fetal deaths, and a variety of teratogenic effects. The compound also caused pseudohermaphroditism in rats treated with approximately 3 times the maximum daily human dose. If this drug must be used during pregnancy, or if the patient becomes pregnant while taking the drug, the patient should be apprised of the potential hazard to the fetus.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Aminoglutethimide in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Aminoglutethimide in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Aminoglutethimide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Aminoglutethimide during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Aminoglutethimide with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Aminoglutethimide with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Aminoglutethimide with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Aminoglutethimide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Aminoglutethimide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Aminoglutethimide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Aminoglutethimide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Aminoglutethimide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Aminoglutethimide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Aminoglutethimide in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Aminoglutethimide in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Aminoglutethimide in the drug label.
Pharmacology
There is limited information regarding Aminoglutethimide Pharmacology in the drug label.
Mechanism of Action
- Cytadren inhibits the enzymatic conversion of cholesterol to ∆5 -pregnenolone, resulting in a decrease in the production of adrenal glucocorticoids, mineralocorticoids, estrogens, and androgens.
- Cytadren blocks several other steps in steroid synthesis, including the C-11, C-18, and C-21 hydroxylations and the hydroxylations required for the aromatization of androgens to estrogens, mediated through the binding of Cytadren to cytochrome P-450 complexes.
- A decrease in adrenal secretion of cortisol is followed by an increased secretion of pituitary adrenocorticotropic hormone (ACTH), which will overcome the blockade of adrenocortical steroid synthesis by Cytadren. The compensatory increase in ACTH secretion can be suppressed by the simultaneous administration of hydrocortisone. Since Cytadren increases the rate of metabolism of dexamethasone but not that of hydrocortisone, the latter is preferred as the adrenal glucocorticoid replacement.
- Although Cytadren inhibits the synthesis of thyroxine by the thyroid gland, the compensatory increase in thyroid-stimulating hormone (TSH) is frequently of sufficient magnitude to overcome the inhibition of thyroid synthesis due to Cytadren. In spite of an increase in TSH, Cytadren has not been associated with increased prolactin secretion.
Note: Cytadren was marketed previously as an anticonvulsant but was withdrawn from marketing for that indication in 1966 because of the effects on the adrenal gland.
Structure
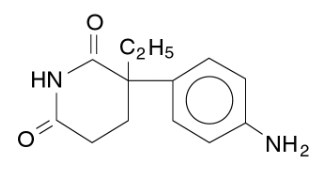
- Cytadren, aminoglutethimide tablets USP, is an inhibitor of adrenocortical steroid synthesis, available as 250-mg tablets for oral administration. Its chemical name is 3-(4-aminophenyl)-3-ethyl-2,6- piperidinedione, and its structural formula is
- Aminoglutethimide USP is a fine, white or creamy white, crystalline powder. It is very slightly soluble in water, and readily soluble in most organic solvents. It forms water- soluble salts with strong acids. Its molecular weight is 232.28. Inactive Ingredients. Cellulose compounds, colloidal silicon dioxide, starch, stearic acid, and talc.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Aminoglutethimide in the drug label.
Pharmacokinetics
- Cytadren is rapidly and completely absorbed after oral administration. In 6 healthy male volunteers, maximum plasma levels of Cytadren averaged 5.9 µg/mL at a median of 1.5 hours after ingestion of two 250-mg tablets. The bioavailability of tablets is equivalent to equal doses given as a solution. After ingestion of a single oral dose, 34%-54% is excreted in the urine as unchanged drug during the first 48 hours, and an additional fraction as the N-acetyl derivative.
- The half-life of Cytadren in normal volunteers given single oral doses averaged 12.5 ± 1.6 hours.
- Upon withdrawal of therapy with Cytadren, the ability of the adrenal glands to synthesize steroid returns, usually within 72 hours.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Aminoglutethimide in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Aminoglutethimide in the drug label.
How Supplied
Storage
There is limited information regarding Aminoglutethimide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Aminoglutethimide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Aminoglutethimide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Aminoglutethimide in the drug label.
Precautions with Alcohol
- Alcohol-Aminoglutethimide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Aminoglutethimide |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Aminoglutethimide |Label Name=Aminoglutethimide11.png
}}
{{#subobject:
|Label Page=Aminoglutethimide |Label Name=Aminoglutethimide11.png
}}