Aminocoumarin: Difference between revisions
m (Protected "Aminocoumarin": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
||
| Line 1: | Line 1: | ||
{{SI}} | {{SI}} | ||
[[Image:Novobiocin.png|thumb|left|[[Skeletal formula]] of [[novobiocin]]]] | [[Image:Novobiocin.png|thumb|left|[[Skeletal formula]] of [[novobiocin]]]] | ||
| Line 30: | Line 30: | ||
[[Category: Antibiotics]] | [[Category: Antibiotics]] | ||
{{WikiDoc Help Menu}} | {{WikiDoc Help Menu}} | ||
{{WS}} | {{WS}} | ||
Latest revision as of 22:04, 8 August 2012
|
WikiDoc Resources for Aminocoumarin |
|
Articles |
|---|
|
Most recent articles on Aminocoumarin Most cited articles on Aminocoumarin |
|
Media |
|
Powerpoint slides on Aminocoumarin |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Aminocoumarin at Clinical Trials.gov Trial results on Aminocoumarin Clinical Trials on Aminocoumarin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Aminocoumarin NICE Guidance on Aminocoumarin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Aminocoumarin Discussion groups on Aminocoumarin Patient Handouts on Aminocoumarin Directions to Hospitals Treating Aminocoumarin Risk calculators and risk factors for Aminocoumarin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Aminocoumarin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
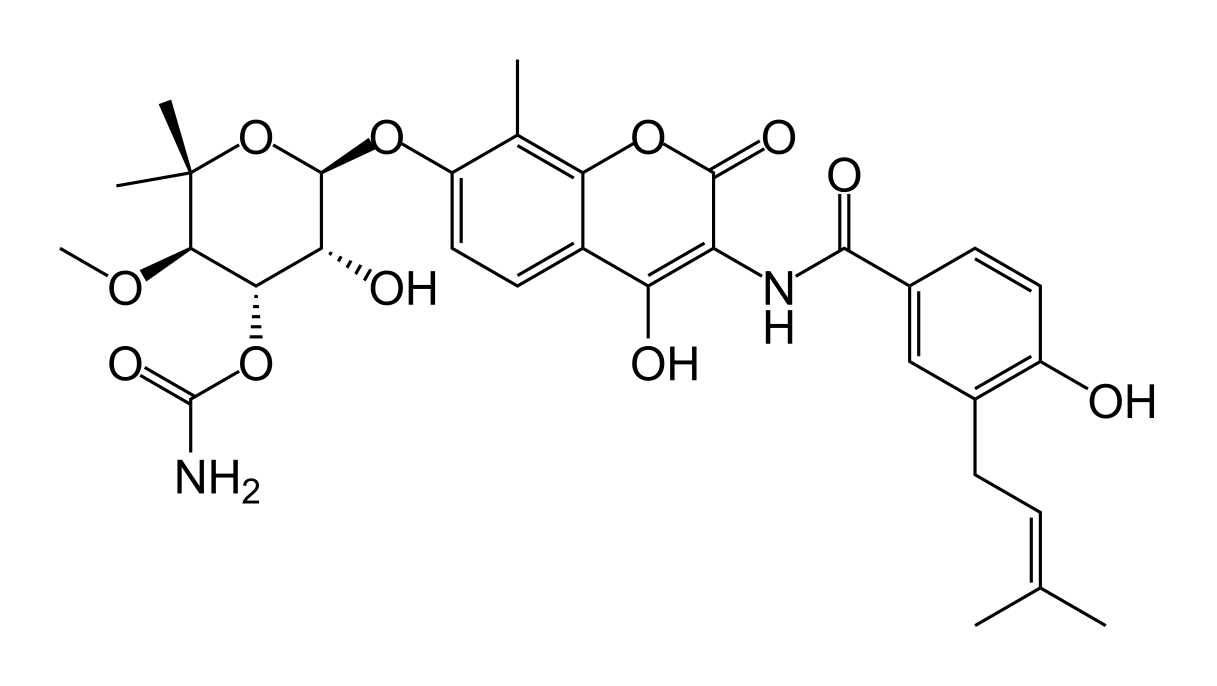
Aminocoumarin is a class of antibiotics which act by an inhibition of the DNA Gyrase enzyme involved in the cell division in bacteria. They are derived from Streptomyces species, whose most well-known representative Streptomyces coelicolor was completely sequenced in 2002.[1] The Aminocoumarin antibiotics include
- Novobiocin, Albamycin® (Pharmacia And Upjohn)
- Coumermycin
- Clorobiocin
Mechanism of action
The Aminocoumarin antibiotics are known inhibitors of DNA gyrase. Antibiotics of the aminocoumarin family exert their therapeutic activity by binding tightly to the B subunit of bacterial DNA gyrase, thereby inhibiting this essential enzyme.[2] They compete with ATP for binding to the B subunit of this enzyme and inhibit the ATP-dependent DNA supercoiling catalysed by gyrase.[3] X-ray crystallography studies have confirmed binding at the ATP-binding site located on the gyrB subunit of DNA gyrase.[4]
Structure
The core of aminocoumarin antibiotics is made up of a 3-Amino-4,7-dihydroxycumarin ring, which is linked e.g. with a sugar in 7-Position and a benzoic acid derivative in 3-Position.
Clorobiocin is a natural antibiotic isolated from several Streptomyces strains and differs from novobiocin in that the methyl group at the 8 position in the coumarin ring of novobiocin is replaced by a chlorine atom, and the carbamoyl at the 3' position of the noviose sugar is substituted by a 5-methyl-2-pyrrolylcarbonyl group.[4]
Clinical use
The clinical use of these antibiotic class has been restricted due to their low water solubility, low activity against gram-negative bacteria[3] and toxiciy in vivo[5].
References
- ↑ Bentley SD, et al. Complete genome sequence of the model actinomycete "Streptomyces coelicolor" A3(2). Nature. 2002 (417)141–147 http://www.nature.com/nature/journal/v417/n6885/abs/417141a.html
- ↑ Galm, Ute, Heller, Stefanie, Shapiro, Stuart, Page, Malcolm, Li, Shu-Ming, Heide, Lutz Antimicrobial and DNA Gyrase-Inhibitory Activities of Novel Clorobiocin Derivatives Produced by Mutasynthesis Antimicrob. Agents Chemother. 2004 48: 1307-1312
- ↑ 3.0 3.1 Maxwell, A., and Lawson, D. M. (2003). The ATP-binding site of type II topoisomerases as a target for antibacterial drugs. Curr Top Med Chem, 3, 283-303.
- ↑ 4.0 4.1 F.T.F. Tsai, O.M. Singh, T.Skarzynski, A.J. Wonacott, S. Weston, A. Tucker, R.A. Pauptit, A.L. Breeze, J.P. Poyser, R. O'Brien et al., The high-resolution crystal structure of a 24-kDa gyrase B fragment from E. coli complexed with one of the most potent coumarin inhibitors, clorobiocin. Proteins 28 (1997), pp. 41–52
- ↑ A. Maxwell, The interaction between coumarin drugs and DNA gyrase. Mol. Microbiol. 9 (1993), pp. 681–686.