Amantadine (Gocovri)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Amantadine (Gocovri) is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Amantadine (Gocovri) in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
(Description)g
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of Amantadine (Gocovri) and IV administrations.
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
| |
| Template:Px | |
Amantadine (Gocovri)
| |
| Systematic (IUPAC) name | |
| Adamantan-1-amine | |
| Identifiers | |
| CAS number | |
| ATC code | N04 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 151.249 g/mol |
| SMILES | & |
| Synonyms | 1-Adamantylamine |
| Pharmacokinetic data | |
| Bioavailability | 86–90%[1] |
| Protein binding | 67%[1] |
| Metabolism | Minimal (mostly to acetyl metabolites)[1] |
| Half life | 10–31 hours[1] |
| Excretion | Urine[1] |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
Prescription Only (S4)(AU) ?(CA) POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Oral |
Mechanism of Action
- The mechanism by which amantadine exerts efficacy in the treatment of dyskinesia in patients with Parkinson’s disease is unknown. Amantadine is a weak uncompetitive antagonist of the NMDA receptor. Amantadine has not been shown to possess direct anticholinergic activity in animal studies; however, it exhibits anticholinergic-like side effects such as dry mouth, urinary retention, and constipation in humans. Amantadine may have direct and indirect effects on dopamine neurons; it exerts dopaminergic-like side effects such as hallucinations and dizziness in humans.
Structure
Pharmacodynamics
- The effect of amantadine on QT prolongation was not studied in a dedicated thorough QT study.
- Alcohol consumption may increase the potential for CNS effects such as dizziness, confusion, lightheadedness, and orthostatic hypotension.
Pharmacokinetics
- Amantadine is an extended release formulation. The pharmacokinetics of amantadine from 68.5 mg, 137 mg, and 274 mg of amantadine are dose proportional in healthy subjects.
Absorption
- After a single dose bedtime administration of amantadine, the median Tmax for plasma amantadine was around 12 hours (range 6-20 hours). The steady-state concentrations of amantadine were achieved 4 days after the dose initiation. The steady-state total exposures (AUC0-tau) were 20-30% higher than after single dose, suggesting an accumulation ratio of 1.2-1.3.
Effect of Food
- A single dose crossover study of amantadine established the lack of effect of high-fat, high-calorie meal on plasma amantadine pharmacokinetics; additionally, administration of entire capsule contents sprinkled on applesauce also did not affect plasma amantadine pharmacokinetics.
Distribution
- The volume of distribution determined after the intravenous administration of amantadine to 15 healthy subjects was 3 to 8 L/kg, suggesting potential extravascular distribution. Amantadine is approximately 67% bound to plasma proteins over a concentration range of 0.1 to 2.0 µg/mL.
Elimination
- In a study with healthy volunteers after oral administration, the apparent plasma clearance of amantadine was estimated to be 0.27 ± 0.094 L/hr/kg (range 0.13 to 0.57 L/hr/kg). Amantadine is primarily excreted unchanged in the urine, and in a study of six healthy volunteers, the ratio of amantadine renal clearance to apparent plasma clearance was 0.79 ± 0.17 (mean ± SD). The mean plasma amantadine half-life at steady-state was approximately 16 hours.
Metabolism
- Eight metabolites of amantadine have been identified in human urine. One metabolite, an N-acetylated compound, was quantified in human urine and accounted for 0-15% of the administered dose in multiple studies. The contribution of this metabolite to efficacy or toxicity is not known.
Excretion
- Amantadine is primarily excreted unchanged in the urine by both glomerular filtration and tubular secretion.
Specific Population
Male/Female Patients
- In an integrated analysis of five studies in healthy volunteers (n=147), the mean total amantadine clearance following administration of amantadine, adjusted for body weight in kilograms, was 1.2 fold higher in males compared to females (95% CI: 1.1, 1.3, P=0.007). No dose adjustment by gender is warranted.
Renal Impairment
- The renal clearance of amantadine is significantly lower in adult patients with moderate or severe renal impairment, compared to healthy adults. Since the renal pathway is a major elimination pathway, impairment in renal function can result in significant accumulation in the plasma, warranting dose adjustment. The impact of renal impairment on dose adjustment was not investigated in a dedicated study.
- Based on PK simulations, the range of the total exposures (AUC0-tau) in subjects with normal renal function (creatinine clearance >90 mL/min/1.73 m2) or mild renal impairment (creatinine clearance between 60 and 89 mL/min/1.73 m2) were comparable for the same dosing regimen. However, patients with moderate renal impairment (creatinine clearance between 30 and 59 mL/min/1.73 m2) had higher exposures relative to patients with normal renal function or mild renal impairment. Severe renal impairment (creatinine clearance between 15 and 29 mL/min/1.73 m2) resulted in even higher total exposures. Dosage adjustment is recommended in patients with moderate and severe renal impairment. Amantadine is contraindicated in patients with end stage renal disease (creatinine clearance less than 15 mL/min/1.73 m2).
- Amantadine is inefficiently removed by hemodialysis.
Drug Interaction Studies
- The in-vitro dissolution-release profiles showed 52% drug release after 45 minutes, and up to 95% after 2 hours, at concentrations of 40% alcohol/0.1N HCl.
- In vitro studies indicate that amantadine has negligible or no inhibitory activity against cellular transporters (P-gp, BCRP, MATE2-K, OAT1, OAT3, OATP1B1, and OATP1B3) at plasma concentrations observed in patients with Parkinson’s disease on an amantadine 274 mg dose.
- In vitro studies in MDCK-II cells demonstrated that amantadine is not a substrate of the anionic transporters OAT1 or OAT3, or the cationic transporter MATE2-K. Amantadine was a poor substrate of the cationic transporters OCT2 and MATE1. Renal elimination of amantadine may be mediated in part by one or more organic cation transporters independent of OCT2. An in vivo study demonstrated that quinidine, a known organic cation transporter inhibitor, reduced amantadine clearance by approximately 33% in humans. The clinical significance is unknown. In vitro studies show that amantadine does not significantly inhibit the enzyme activity of drug metabolizing cytochrome P450 isoforms (CYP1A2, 2B6, 2C19, 2C8, 2C9, 2D6, 2E1, 3A4, and 3A5).
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- Animal studies designed to evaluate the carcinogenic potential of amantadine have not been conducted.
Mutagenesis
- Amantadine was negative for genotoxicity in in vitro (Ames and mammalian cell [Chinese Hamster ovary and human peripheral blood lymphocytes]) assays in the presence or absence of metabolic activation and in an in vivo mouse bone marrow micronucleus assay.
Impairment of Fertility
- The effects of amantadine on fertility have not been adequately tested in a study in animals conducted according to current standards. In a reproduction study reported in the literature, oral administration of amantadine to male and female rats at a dose of 32 mg/kg/day resulted in impaired fertility. The no-effect dose for adverse effects on fertility (10 mg/kg/day) is less than the recommended human dose of 274 mg/day on a mg/m2 basis.
Clinical Studies
Overview of Studies
- Amantadine for the treatment of dyskinesia in patients with Parkinson’s disease was assessed in two randomized, double-blind, placebo-controlled efficacy trials: Study 1 and Study 2. Key inclusion criteria in both studies included at least 1 hour of troublesome dyskinesia time during the day and at least mild functional impact because of dyskinesia.
- Study 1 was conducted in 121 (modified Intention to Treat (mITT) population) Parkinson’s disease patients with dyskinesia in the United States (US) and Canada. The duration of treatment in Study 1 was up to 25 weeks. Study 2 was conducted in 75 (mITT population) patients with dyskinesia in the US, Germany, France, Spain, and Austria. The duration of treatment was 13 weeks.
- In both studies, the primary efficacy endpoint was the change in total score of the Unified Dyskinesia Rating Scale (UDysRS) between baseline and Week 12. Key secondary endpoints derived from a Parkinson’s disease home diary included changes from baseline to Week 12 in ON time without troublesome dyskinesia and OFF time.
Study Population
- In Study 1 and Study 2, the mean age of patients at the time of Parkinson’s disease diagnosis was 55 years (range: 29-75 years).
- At baseline, patients had a mean UDysRS total score of 40.1 (range: 8-76), a mean duration of ON time without troublesome dyskinesia (Parkinson’s disease home diary) of 8.4 hours (range: 0-15.3), and a mean duration of OFF time of 2.8 hours (range: 0-9.5).
- Patients in Study 1 and Study 2 were treated with a stable dose of levodopa, with 32% of patients on levodopa monotherapy. Patients were also treated with concomitant dopamine agonists (54%) and/or MAO-B inhibitors (44%).
Study 1
- In Study 1, a significant decrease in mean UDysRS total score (reduction in dyskinesia) was observed at Week 12 in patients treated with amantadine, compared with placebo.
Study 2
- In Study 2, a significant decrease in mean UDysRS total score (reduction in dyskinesia) was observed at Week 12 in patients treated with amantadine, compared with placebo (TABLE 2).
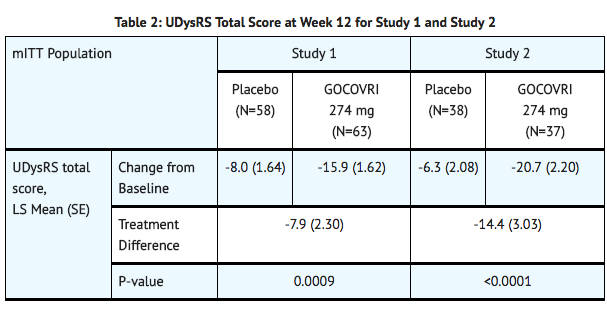
- In Study 1 and Study 2, there was a significant increase in ON time without troublesome dyskinesia, and a significant decrease in OFF time between baseline and Week 12 in patients treated with amantadine, compared with placebo (TABLE 3).
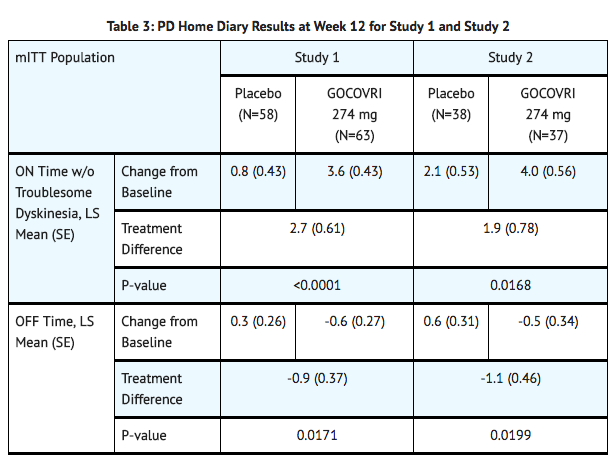
How Supplied
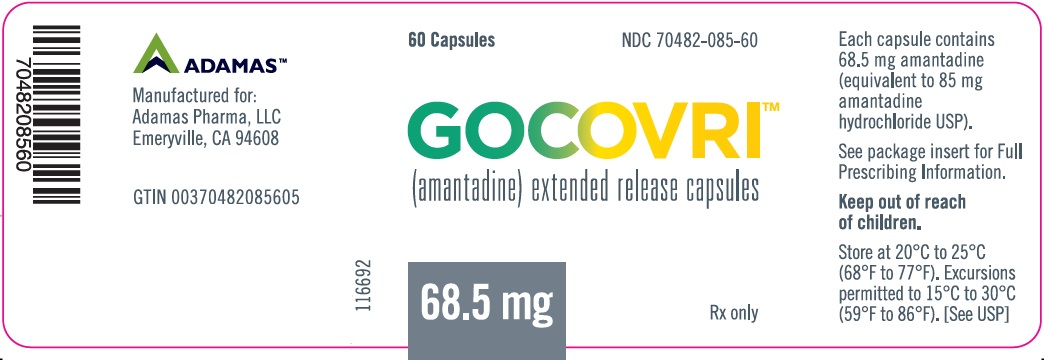
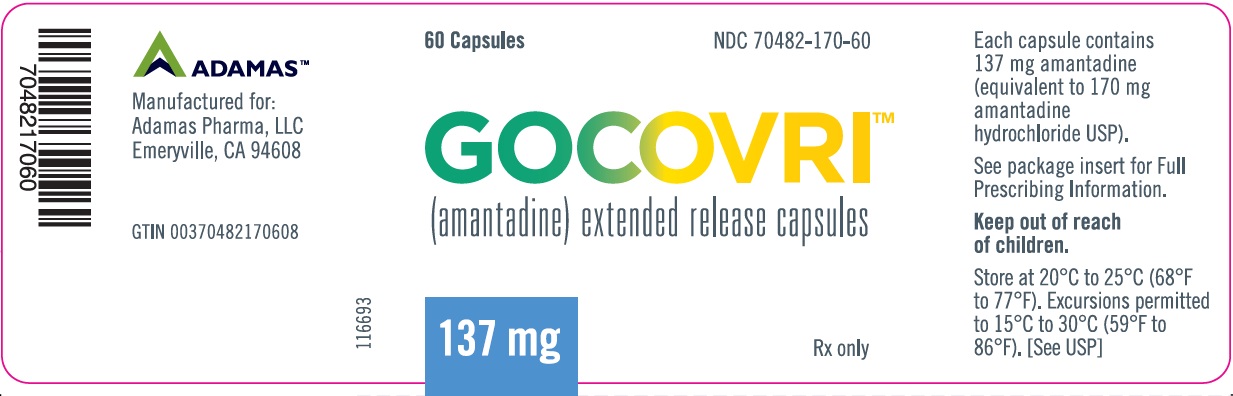
- Amantadine is supplied as extended release capsules in the following configurations:
- The 68.5 mg capsule is a white opaque size #2 capsule, with black printing of ‘ADAMAS’ on front and ‘85’ on back of the cap and three black bands printed on body of capsule.
- 60 count bottles NDC# 70482-085-60
- The 137 mg capsule is a light blue opaque size #0 capsule, with black printing of ‘ADAMAS’ on front and ‘170’ on back of the cap and three black bands printed on body of capsule.
- 60 count bottles NDC# 70482-170-60
Storage
- Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
Images
Drug Images
{{#ask: Page Name::Amantadine (Gocovri) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Amantadine (Gocovri) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Patient Information).
Administration
- Instruct patients and caregivers that amantadine capsules should be swallowed whole and can be administered with or without food. Alternatively, amantadine capsules may be opened and the contents sprinkled on applesauce; the entire contents should be consumed immediately without chewing. Advise patients to speak to their healthcare provider before discontinuation of amantadine.
Falling Asleep During Activities of Daily Living
- Advise patients that sleepiness and fatigue that have been reported with amantadine and patients treated with Parkinson’s disease medications have reported falling asleep while engaged in activities of daily living. These adverse reactions may affect some patients’ ability to drive and operate machinery safely.
Suicidality and Depression
- Instruct patients, family members, and caregivers to notify their healthcare provider if depressed mood, depression, changes in behavior or thinking, and suicidal ideation or behavior develop during treatment.
Hallucinations/Psychotic Behavior
- Inform patients and caregivers that hallucinations and paranoia can occur while taking amantadine. Tell patients to report unreal visions, sounds, or sensations or other psychotic behavior to their healthcare provider promptly should they develop.
Dizziness and Orthostatic Hypotension
- An increased incidence of dizziness, orthostatic hypotension, and syncope was observed with administration of amantadine. Caution patients against standing rapidly after sitting or lying down, especially if they have been doing so for prolonged periods and especially at the initiation of treatment with amantadine.
Withdrawal-Emergent Hyperpyrexia and Confusion
- Advise patients to contact their healthcare provider before stopping amantadine. Tell patients to inform their healthcare provider if they develop withdrawal symptoms such as fever, confusion, or severe muscle stiffness.
Impulse Control/Compulsive Disorders
- Inform patients of the potential for experiencing intense urges to gamble, increased sexual urges, intense urges to spend money, binge eating, and other intense urges and the inability to control these urges while taking one or more of the medications that increase central dopaminergic tone, that are generally used for the treatment of Parkinson’s disease.
Drug Interactions
- Certain medications can cause an interaction with amantadine. Advise patients and/or caregivers to inform their healthcare provider of all the medicines the patient is taking, including over-the-counter medicines, dietary supplements, and herbal products. Inform patients that live influenza vaccines and consumption of alcohol are not recommended during treatment with amantadine.

Precautions with Alcohol
Alcohol-Amantadine (Gocovri) interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Gocovri
Look-Alike Drug Names
There is limited information regarding Amantadine (Gocovri) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.