DIGOXIN injection dosage and administration
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Abdurahman Khalil, M.D. [2]
For patient information, click here.
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosing and Administration Information
In selecting a digoxin injection dosing regimen, it is important to consider factors that affect digoxin blood levels (e.g., body weight, age, renal function, concomitant drugs) since toxic levels of digoxin are only slightly higher than therapeutic levels. Dosing can be either initiated with a loading dose followed by maintenance dosing if rapid titration is desired or initiated with maintenance dosing without a loading dose.
Parenteral administration of digoxin should be used only when the need for rapid digitalization is urgent or when the drug cannot be taken orally. Intramuscular injection can lead to severe pain at the injection site, thus intravenous administration is preferred. If the drug must be administered by the intramuscular route, it should be injected deep into the muscle followed by massage. For adults, no more than 500 mcg of digoxin injection should be injected into a single site. For pediatric patients, no more than 200 mcg of digoxin injection should be injected into a single site.
Administer the dose over a period of 5 minutes or longer and avoid bolus administration to prevent systemic and coronary vasoconstriction. Mixing of digoxin injection with other drugs in the same container or simultaneous administration in the same intravenous line is not recommended.
Digoxin injection can be administered undiluted or diluted with a 4-fold or greater volume of Sterile Water for Injection, 0.9% Sodium Chloride Injection, or 5% Dextrose Injection. The use of less than a 4-fold volume of diluent could lead to precipitation of the digoxin. Immediate use of the diluted product is recommended.
If tuberculin syringes are used to measure very small doses do not flush with the parenteral solution after its contents are expelled into an indwelling vascular catheter to avoid overadministration of digoxin.
Consider interruption or reduction in digoxin injection dose prior to electrical cardioversion [see Warnings and Precautions (5.4)].
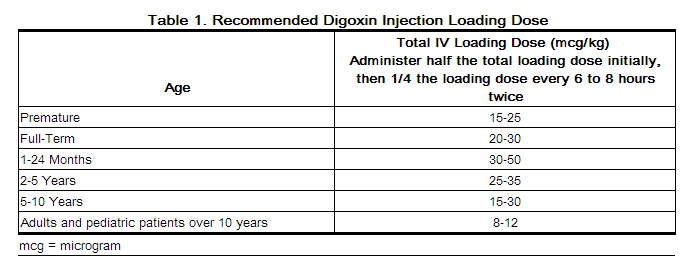
2.2 Loading Dosing Regimen in Adults and Pediatric Patients
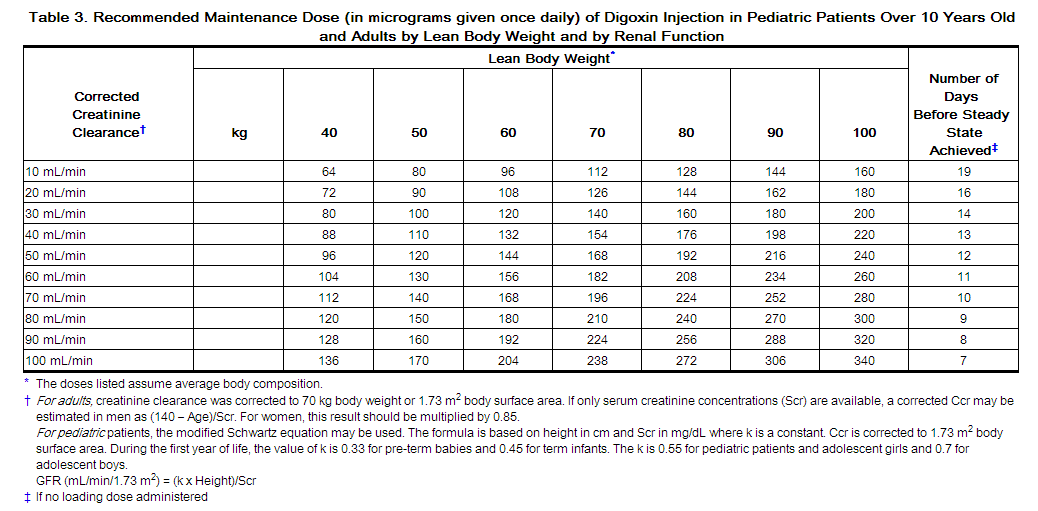
2.3 Maintenance Dosing in Adults and Pediatric Patients Over 10 Years Old
The maintenance dose is based on lean body weight, renal function, age, and concomitant products [see Clinical Pharmacology (12.3)].
The recommended starting maintenance doses in adults and pediatric patients over 10 years old with normal renal function are given in Table 2. Doses may be increased every 2 weeks according to clinical response, serum drug levels, and toxicity.
Table 3 provides the recommended (once daily) maintenance dose for adults and pediatric patients over 10 years old (to be given once daily) according to lean body weight and renal function. The doses are based on studies in adult patients with heart failure. Alternatively, the maintenance dose may be estimated by the following formula (peak body stores lost each day through elimination):
Total Maintenance Dose = Loading Dose (i.e., Peak Body Stores) x % Daily Loss/100
(% Daily Loss = 14 + Creatinine clearance/5)
Reduce the dose of digoxin injection in patients whose lean weight is an abnormally small fraction of their total body mass because of obesity or edema.
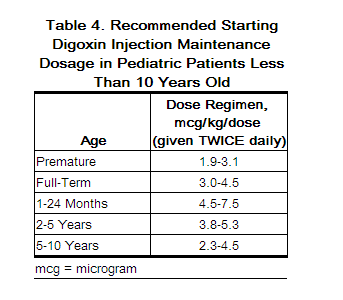
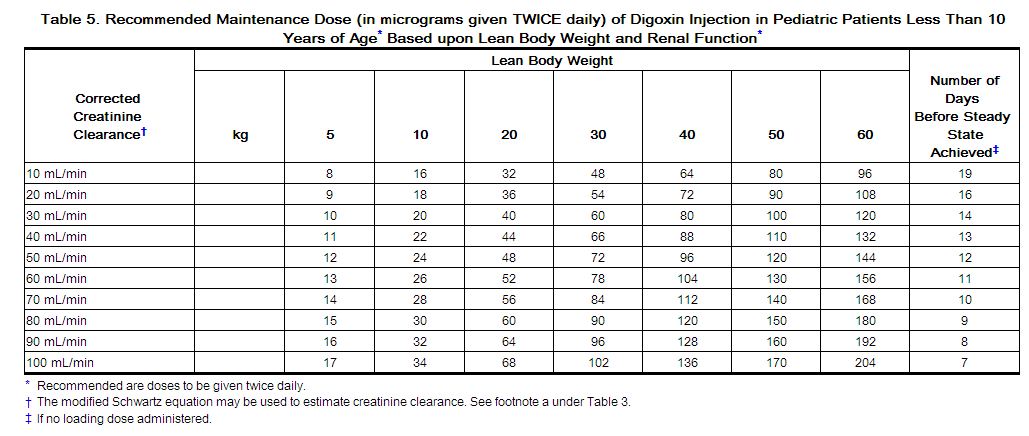
2.4 Maintenance Dosing in Pediatric Patients Less Than 10 Years Old
The starting maintenance dose for heart failure in pediatric patients less than 10 years old is based on lean body weight, renal function, age, and concomitant products [see Clinical Pharmacology (12.3)]. The recommended starting maintenance doses for pediatric patients are given in Table 4. These recommendations assume the presence of normal renal function.
Table 5 provides average daily maintenance dose requirements for pediatric patients less than 10 years old (to be given twice daily) with heart failure based on age, lean body weight, and renal function.
2.5 Monitoring to Assess Safety, Efficacy, and Therapeutic Blood Levels
Monitor for signs and symptoms of digoxin toxicity and clinical response. Adjust dose based on toxicity, efficacy, and blood levels.
Serum digoxin levels less than 0.5 ng/mL have been associated with diminished efficacy, while levels above 2 ng/mL have been associated with increased toxicity without increased benefit.
Interpret the serum digoxin concentration in the overall clinical context, and do not use an isolated measurement of serum digoxin concentration as the basis for increasing or decreasing the digoxin injection dose. Serum digoxin concentrations may be falsely elevated by endogenous digoxin-like substances [see Drug Interactions (7.4)]. If the assay is sensitive to these substances, consider obtaining a baseline digoxin level before starting digoxin injection and correct post-treatment values by the reported baseline level.
Obtain serum digoxin concentrations just before the next scheduled digoxin injection dose or at least 6 hours after the last dose. The digoxin concentration is likely to be 10 to 25% lower when sampled right before the next dose (24 hours after dosing) compared to sampling 8 hours after dosing (using once-daily dosing). However, there will be only minor differences in digoxin concentrations using twice daily dosing whether sampling is done at 8 or 12 hours after a dose.
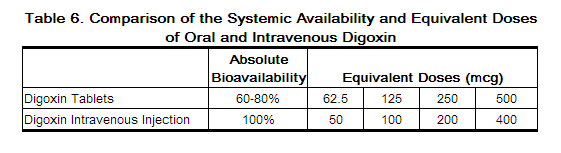
2.6 Switching from Intravenous Digoxin to Oral Digoxin
When switching from intravenous to oral digoxin formulations, make allowances for differences in bioavailability when calculating maintenance dosages (see Table 6).
References
http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=58f45aba-ff6f-43cc-bb88-be40a9f7beda