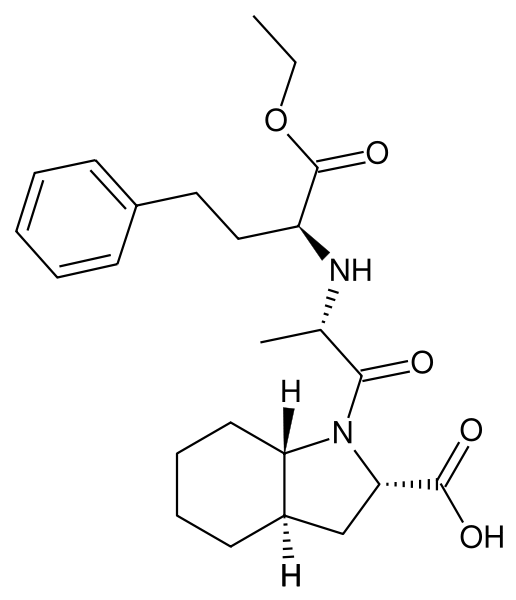
Trandolapril
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Amr Marawan, M.D. [2]
For patient information about Trandolapril, click here.
Synonyms / Brand Names: MAVIK®
Overview
Trandolapril is an ACE inhibitor used to treat high blood pressure, it may also be used to treat other conditions. It is marketed by Abbott Laboratories with the brand name Mavik®.
Category
Antihypertensive Agents, ACE Inhibitors
FDA Package Insert
MAVIK (Trandolapril hydrochloride) tablet
Indications and Usage | Dosage and Administration | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Overdosage | Description | Clinical Pharmacology | Clinical Studies | How Supplied/Storage and Handling | Patient Counseling Information | Labels and Packages
 |
Interactions with Alcohol
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | Trandolapril 80% (independent of concentration) Trandolaprilat 65 to 94% (concentration-dependent) |
| Metabolism | Hepatic |
| Elimination half-life | 6 hours (trandolapril) 10 hours (trandolaprilat) |
| Excretion | Fecal and renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C24H34N2O5 |
| Molar mass | 430.537 g/mol |
Categories:
- Pages with script errors
- Cardiovascular Drugs
- Drugs
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes