Carteolol description
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Description
Ocupress® (carteolol hydrochloride ophthalmic solution), 1%, is a nonselective beta-adrenoceptor blocking agent for ophthalmic use.
The chemical name for carteolol hydrochloride is (±)–5–[3–[(1,1–dimethylethyl) amino]–2 hydroxypropoxy]–3, 4–dihydro–2(1H)–quinolinone monohydrochloride. The structural formula is as follows:
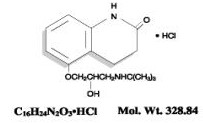 |
Each mL contains 10 mg carteolol HCl and the inactive ingredients – Benzalkonium chloride 0.05 mg (0.005%) as a preservative; sodium chloride; sodium phosphate, dibasic; sodium phosphate, monobasic; and water for injection, USP. The product has a pH of 6.2 to 7.2
References
- ↑ "ZEBETA (BISOPROLOL FUMARATE) TABLET [DURAMED PHARMACEUTICALS, INC.]". Retrieved 4 February 2014.