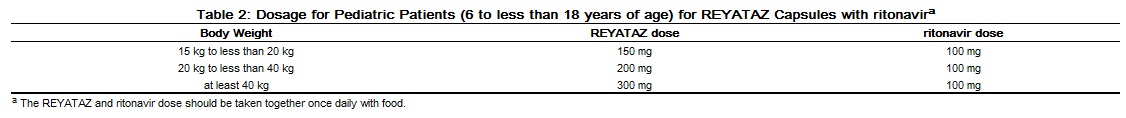Atazanavir dosage and administration
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Mohamed Moubarak, M.D. [2]
Dosage and Administration
General Dosing Recommendations:
- REYATAZ Capsules must be taken with food.
- Do not open the capsules.
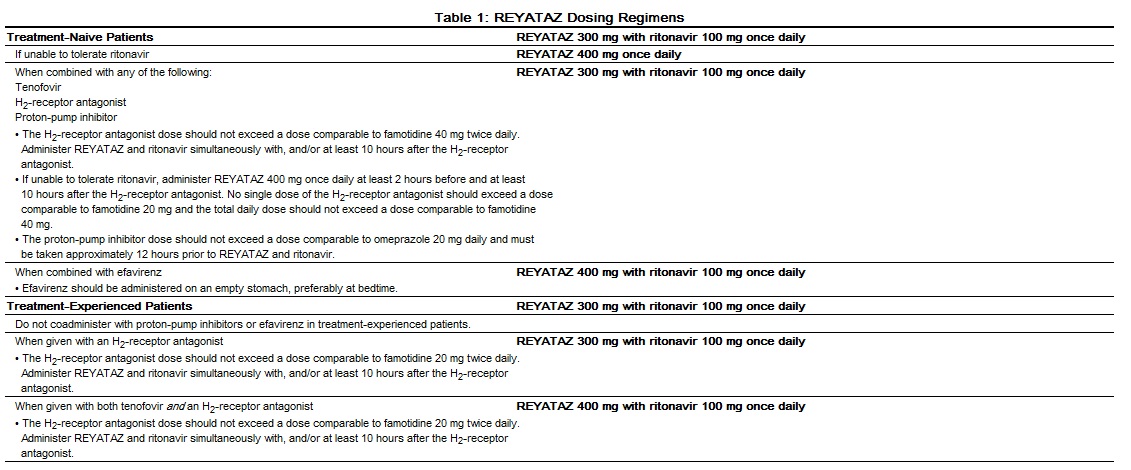
- The recommended oral dosage of REYATAZ depends on the treatment history of the patient and the use of other coadministered drugs. When coadministered with H2-receptor antagonists or proton-pump inhibitors, dose separation may be required.
- When coadministered with didanosine buffered or enteric-coated formulations, REYATAZ should be given (with food) 2 hours before or 1 hour after didanosine.
- REYATAZ without ritonavir is not recommended for treatment-experienced adult or pediatric patients with prior virologic failure.
- Efficacy and safety of REYATAZ with ritonavir in doses greater than 100 mg once daily have not been established. The use of higher ritonavir doses might alter the safety profile of atazanavir (cardiac effects, hyperbilirubinemia) and, therefore, is not recommended. Prescribers should consult the complete prescribing information for NORVIR® (ritonavir) when using this agent.
Recommended Adult Dosage
Table 1 summarizes the recommended REYATAZ dosing regimen in adults. All REYATAZ dosing regimens are to be administered as a single dose with food.
Recommended Pediatric Dosage
The recommended daily dosage of REYATAZ for pediatric patients (6 to less than 18 years of age) is based on body weight and should not exceed the recommended adult dosage. REYATAZ Capsules must be taken with food. The data are insufficient to recommend dosing of REYATAZ for any of the following: (1) patients less than 6 years of age, (2) without ritonavir in any pediatric patient less than 13 years of age, and (3) patients less than 40 kg receiving concomitant tenofovir, H2-receptor antagonists, or proton-pump inhibitors.
The recommended dosage of REYATAZ with ritonavir in pediatric patients at least 6 years of age is shown in Table 2.
For treatment-naive patients at least 13 years of age and at least 40 kg, who are unable to tolerate ritonavir, the recommended dose is REYATAZ 400 mg (without ritonavir) once daily with food. For patients at least 13 years of age and at least 40 kg receiving concomitant tenofovir, H2-receptor antagonists, or proton-pump inhibitors, REYATAZ should not be administered without ritonavir.
Pregnancy
Dosing During Pregnancy and the Postpartum Period:
REYATAZ should not be administered without ritonavir. REYATAZ should only be administered to pregnant women with HIV-1 strains susceptible to atazanavir. For pregnant patients, no dose adjustment is required for REYATAZ with the following exceptions: For treatment-experienced pregnant women during the second or third trimester, when REYATAZ is coadministered with either an H2-receptor antagonist or tenofovir, REYATAZ 400 mg with ritonavir 100 mg once daily is recommended. There are insufficient data to recommend a REYATAZ dose for use with both an H2-receptor antagonist and tenofovir in treatment-experienced pregnant women.
No dose adjustment is required for postpartum patients. However, patients should be closely monitored for adverse events because atazanavir exposures could be higher during the first 2 months after delivery.
Renal Impairment
For patients with renal impairment, including those with severe renal impairment who are not managed with hemodialysis, no dose adjustment is required for REYATAZ. Treatment-naive patients with end stage renal disease managed with hemodialysis should receive REYATAZ 300 mg with ritonavir 100 mg. REYATAZ should not be administered to HIV-treatment-experienced patients with end stage renal disease managed with hemodialysis.
Hepatic Impairment
REYATAZ should be used with caution in patients with mild-to-moderate hepatic impairment. For patients with moderate hepatic impairment (Child-Pugh Class B) who have not experienced prior virologic failure, a dose reduction to 300 mg once daily should be considered. REYATAZ should not be used in patients with severe hepatic impairment (Child-Pugh Class C). REYATAZ/ritonavir has not been studied in subjects with hepatic impairment and is not recommended.[1]
References
- ↑ "REYATAZ (ATAZANAVIR SULFATE) CAPSULE, GELATIN COATED [E.R. SQUIBB & SONS, L.L.C.]". Text " accessdate" ignored (help)
Adapted from the FDA Package Insert.