Cefixime dosage and administration
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
Dosage and Administration
Adults
The recommended dose of cefixime is 400 mg daily. This may be given as a 400 mg tablet or capsule daily or the 400 mg tablet may be split and given as one half tablet every 12 hours. For the treatment of uncomplicated cervical/urethral gonococcal infections, a single oral dose of 400 mg is recommended. The capsule and tablet may be administered without regard to food.
In the treatment of infections due to Streptococcus pyogenes, a therapeutic dosage of cefixime should be administered for at least 10 days.
Pediatric Patients (6 months or older)
The recommended dose is 8 mg/kg/day of the suspension. This may be administered as a single daily dose or may be given in two divided doses, as 4 mg/kg every 12 hours.
Note: A suggested dose has been determined for each pediatric weight range. Refer to Table 1. Ensure all orders that specify a dose in milliliters include a concentration, because Suprax for oral suspension is available in three different concentrations (100 mg/5 mL, 200 mg/5 mL, and 500 mg/5 mL).
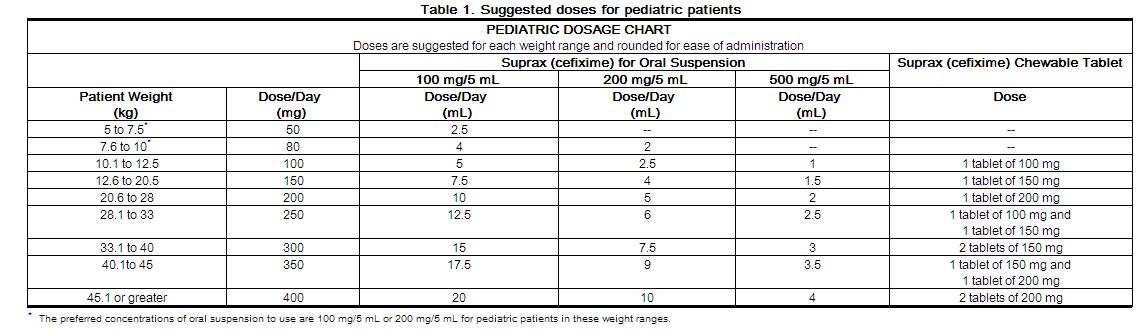 |
Children weighing more than 45 kg or older than 12 years should be treated with the recommended adult dose. Suprax (cefixime) Chewable Tablets must be chewed or crushed before swallowing.
Otitis media should be treated with the chewable tablets or suspension. Clinical trials of otitis media were conducted with the chewable tablets or suspension, and the chewable tablets or suspension results in higher peak blood levels than the tablet when administered at the same dose.
Therefore, the tablet or capsule should not be substituted for the chewable tablets or suspension in the treatment of otitis media.
In the treatment of infections due to Streptococcus pyogenes, a therapeutic dosage of cefixime should be administered for at least 10 days.
Renal Impairment
Suprax may be administered in the presence of impaired renal function. Normal dose and schedule may be employed in patients with creatinine clearances of 60 mL/min or greater. Refer to Table 2 for dose adjustments for adults with renal impairment. Neither hemodialysis nor peritoneal dialysis removes significant amounts of drug from the body.
 |
Reconstitution Directions for Oral Suspension
 |
After reconstitution, the suspension may be kept for 14 days either at room temperature, or under refrigeration, without significant loss of potency. Keep tightly closed. Shake well before using. Discard unused portion after 14 days.[1]
References
Adapted from the FDA Package Insert.