Oxacillin sodium dosage and administration
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Dosage and Administration
The penicillinase-resistant penicillins are available for oral administration and for intramuscular and intravenous injection. The sodium salts of methicillin, oxacillin, and nafcillin may be administered parenterally and the sodium salts of cloxacillin, dicloxacillin, oxacillin, and nafcillin are available for oral use.
Bacteriologic studies to determine the causative organisms and their susceptibility to oxacillin should always be performed. Duration of therapy varies with the type of severity of infection as well as the overall condition of the patient; therefore, it should be determined by the clinical and bacteriological response of the patient. In severe staphylococcal infections, therapy with oxacillin should be continued for at least 14 days. Therapy should be continued for at least 48 hours after the patient has become afebrile, asymptomatic, and cultures are negative. Treatment of endocarditis and osteomyelitis may require a longer duration of therapy.
Concurrent administration of oxacillin and probenecid increases and prolongs serum penicillin levels. Probenecid decreases the apparent volume of distribution and slows the rate of excretion by competitively inhibiting renal tubular secretion of penicillin. Penicillin-probenecid therapy is generally limited to those infections where very high serum levels of penicillin are necessary.
Oral preparations of the penicillinase-resistant penicillins should not be used as initial therapy in serious, life-threatening infections (see PRECAUTIONS-General). Oral therapy with the penicillinase-resistant penicillins may be used to follow-up the previous use of a parenteral agent as soon as the clinical condition warrants. For intramuscular gluteal injections, care should be taken to avoid sciatic nerve injury. With intravenous administration, particularly in elderly patients, care should be taken because of the possibility of thrombophlebitis.
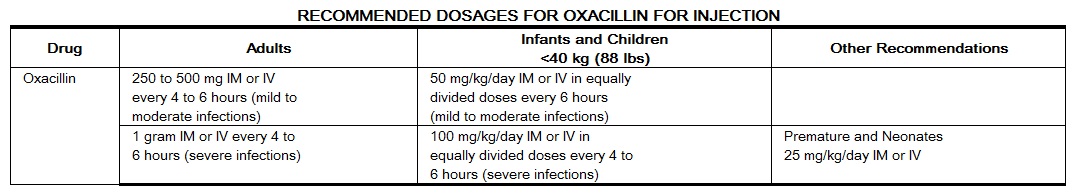
|
References
Adapted from the FDA Package Insert.
- ↑ "OXACILLIN (OXACILLIN SODIUM) INJECTION, POWDER, FOR SOLUTION [AUROMEDICS PHARMA LLC]". Text " accessdate" ignored (help)